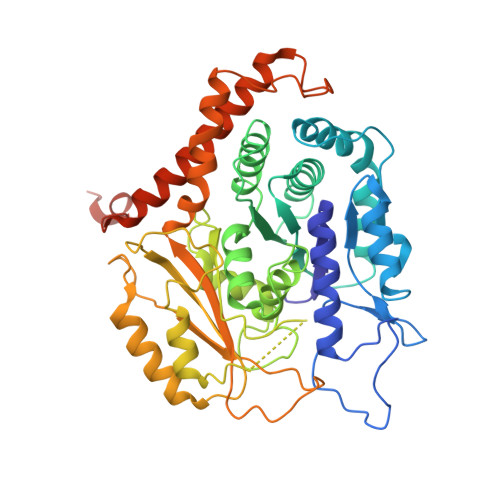
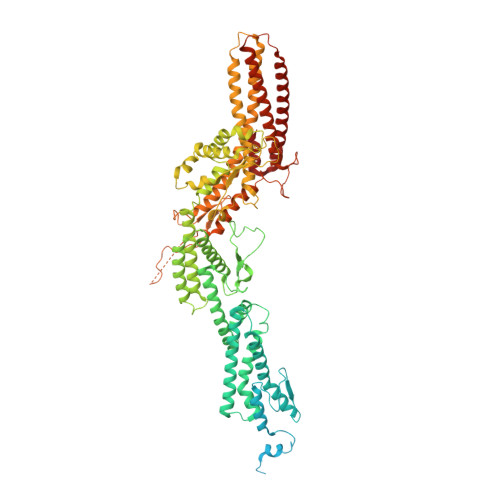
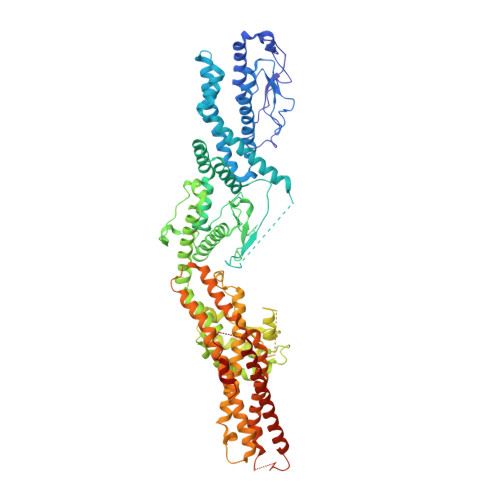
CM1-driven assembly and activation of yeast gamma-tubulin small complex underlies microtubule nucleation.
Brilot, A.F., Lyon, A.S., Zelter, A., Viswanath, S., Maxwell, A., MacCoss, M.J., Muller, E.G., Sali, A., Davis, T.N., Agard, D.A.(2021) Elife 10
- PubMed: 33949948
- DOI: https://doi.org/10.7554/eLife.65168
- Primary Citation of Related Structures:
7M2W, 7M2X, 7M2Y, 7M2Z, 7M3P - PubMed Abstract:
Microtubule (MT) nucleation is regulated by the γ-tubulin ring complex (γTuRC), conserved from yeast to humans. In Saccharomyces cerevisiae , γTuRC is composed of seven identical γ-tubulin small complex (γTuSC) sub-assemblies, which associate helically to template MT growth. γTuRC assembly provides a key point of regulation for the MT cytoskeleton. Here, we combine crosslinking mass spectrometry, X-ray crystallography, and cryo-EM structures of both monomeric and dimeric γTuSCs, and open and closed helical γTuRC assemblies in complex with Spc110p to elucidate the mechanisms of γTuRC assembly. γTuRC assembly is substantially aided by the evolutionarily conserved CM1 motif in Spc110p spanning a pair of adjacent γTuSCs. By providing the highest resolution and most complete views of any γTuSC assembly, our structures allow phosphorylation sites to be mapped, surprisingly suggesting that they are mostly inhibitory. A comparison of our structures with the CM1 binding site in the human γTuRC structure at the interface between GCP2 and GCP6 allows for the interpretation of significant structural changes arising from CM1 helix binding to metazoan γTuRC.
Organizational Affiliation:
Department of Biochemistry and Biophysics, University of California at San Francisco, San Francisco, United States.