Structure-Based Design of Inhibitors of the m6A-RNA Writer Enzyme METTL3
Bedi, R.K., Huang, D., Li, Y., Caflisch, A.(2023) ACS Bio Med Chem Au
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2023) ACS Bio Med Chem Au
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| N6-adenosine-methyltransferase catalytic subunit | 246 | Homo sapiens | Mutation(s): 0 Gene Names: METTL3, MTA70 EC: 2.1.1.348 | 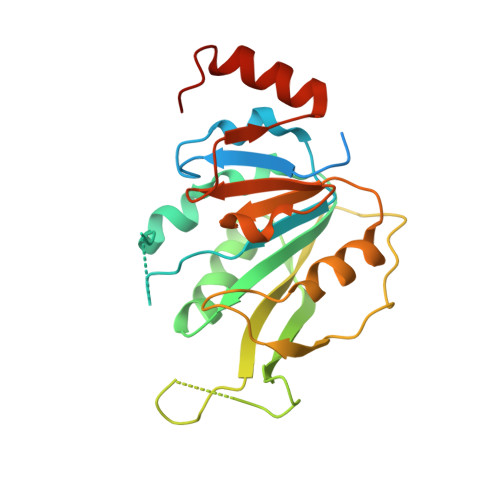 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q86U44 (Homo sapiens) Explore Q86U44 Go to UniProtKB: Q86U44 | |||||
PHAROS: Q86U44 GTEx: ENSG00000165819 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q86U44 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| N6-adenosine-methyltransferase non-catalytic subunit | 290 | Homo sapiens | Mutation(s): 0 Gene Names: METTL14, KIAA1627 | 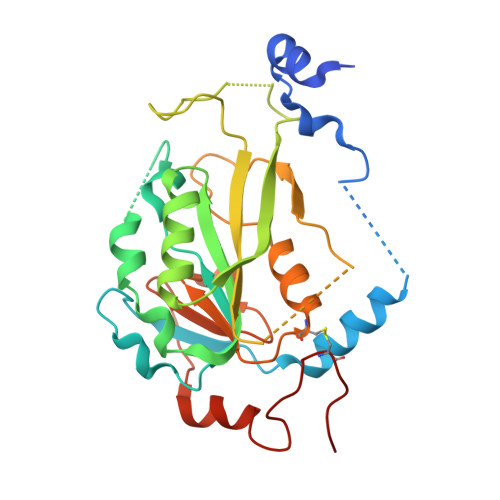 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9HCE5 (Homo sapiens) Explore Q9HCE5 Go to UniProtKB: Q9HCE5 | |||||
PHAROS: Q9HCE5 GTEx: ENSG00000145388 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9HCE5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 0AE (Subject of Investigation/LOI) Query on 0AE | C [auth A] | (3~{R})-1-[6-[(phenylmethyl)amino]pyrimidin-4-yl]-3-[[[6-[[(3~{S})-3-propan-2-yl-2-azoniaspiro[3.3]heptan-2-yl]methyl]naphthalen-1-yl]amino]methyl]piperidin-3-ol C37 H47 N6 O DAMVLLAQUZPVCW-YBZKQSBQSA-O |  | ||
| ACT Query on ACT | D [auth B] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 63.9 | α = 90 |
| b = 63.9 | β = 90 |
| c = 225.09 | γ = 120 |
| Software Name | Purpose |
|---|---|
| XDS | data reduction |
| XSCALE | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Swiss National Science Foundation | Switzerland | 310030B_189363 |