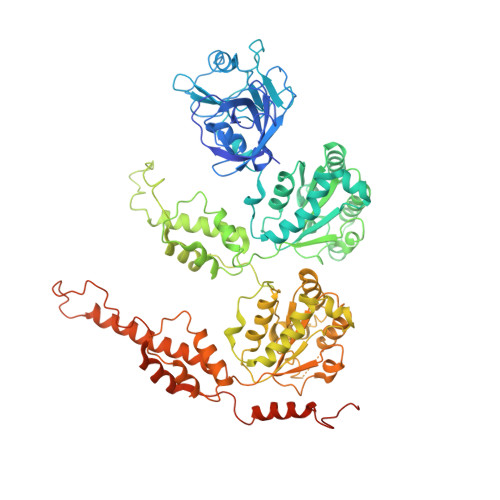
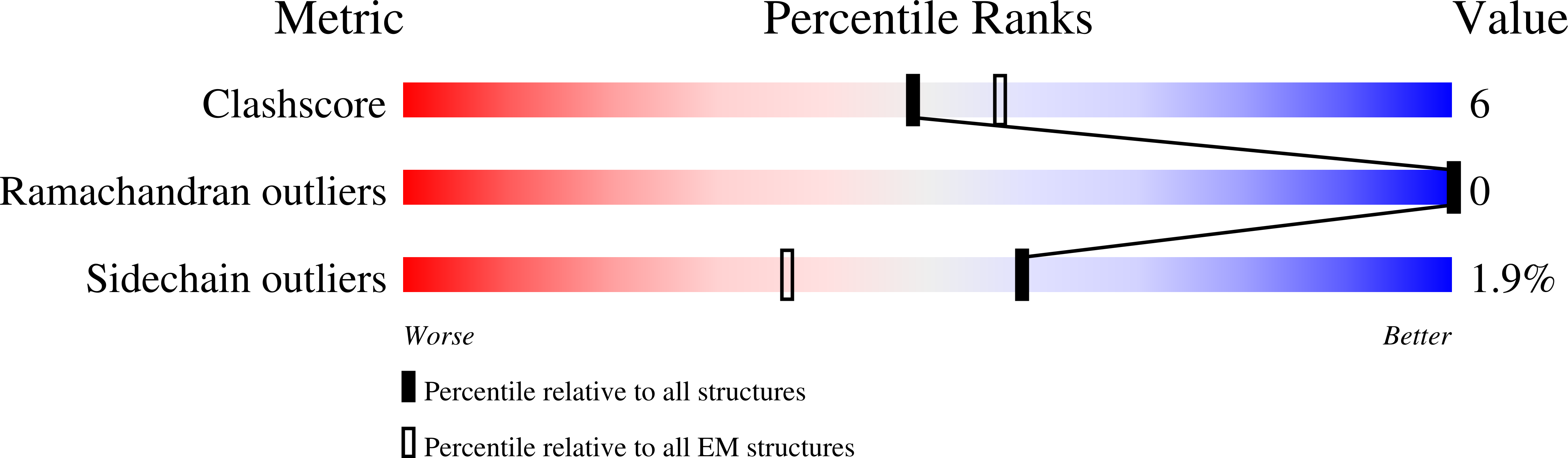Cryo-electron microscopy structures of VCP/p97 reveal a new mechanism of oligomerization regulation.
Yu, G., Bai, Y., Li, K., Amarasinghe, O., Jiang, W., Zhang, Z.Y.(2021) iScience 24: 103310-103310
- PubMed: 34765927
- DOI: https://doi.org/10.1016/j.isci.2021.103310
- Primary Citation of Related Structures:
7K56, 7K57, 7K59 - PubMed Abstract:
VCP/p97 is an evolutionarily conserved AAA+ ATPase important for cellular homeostasis. Previous studies suggest that VCP predominantly exists as a homohexamer. Here, we performed structural and biochemical characterization of VCP dodecamer, an understudied state of VCP. The structure revealed an apo nucleotide status that has rarely been captured, a tail-to-tail assembly of two hexamers, and the up-elevated N-terminal domains akin to that seen in the ATP-bound hexamer. Further analyses elucidated a nucleotide status-dependent dodecamerization mechanism, where nucleotide dissociation from the D2 AAA domains induces and promotes VCP dodecamerization. In contrast, nucleotide-free D1 AAA domains are associated with the up-rotation of N-terminal domains, which may prime D1 for ATP binding. These results therefore reveal new nucleotide status-dictated intra- and interhexamer conformational changes and suggest that modulation of D2 domain nucleotide occupancy may serve as a mechanism in controlling VCP oligomeric states.
Organizational Affiliation:
Departments of Medicinal Chemistry and Molecular Pharmacology and of Chemistry, Center for Cancer Research, and Institute for Drug Discovery, Purdue University, 720 Clinic Drive, West Lafayette, IN 47907, USA.