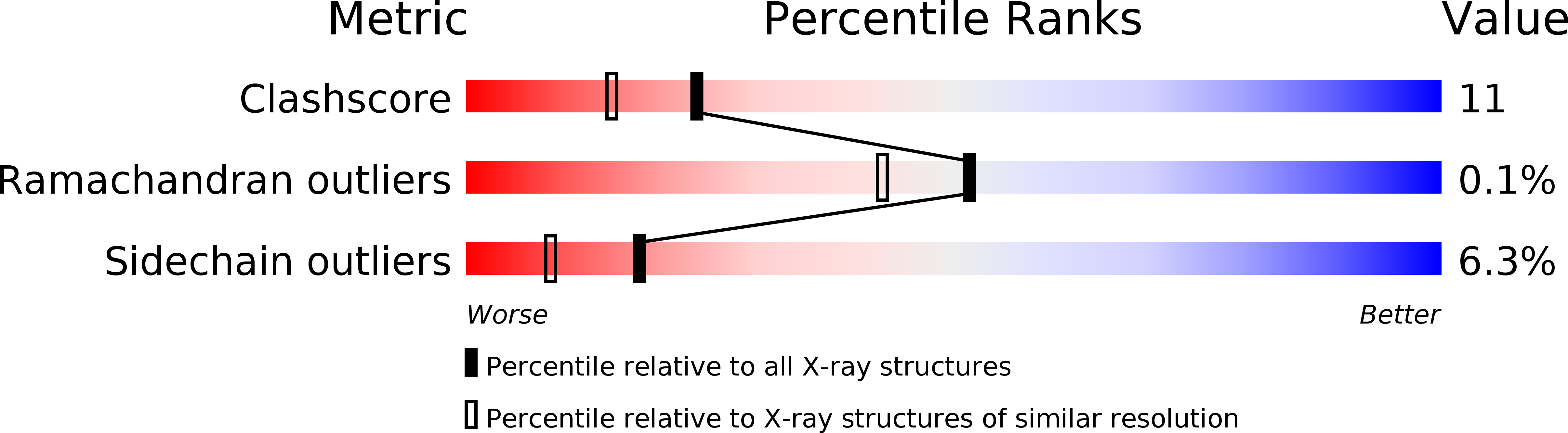Movement of the biotin carboxylase B-domain as a result of ATP binding.
Thoden, J.B., Blanchard, C.Z., Holden, H.M., Waldrop, G.L.(2000) J Biol Chem 275: 16183-16190
- PubMed: 10821865
- DOI: https://doi.org/10.1074/jbc.275.21.16183
- Primary Citation of Related Structures:
1DV1, 1DV2 - PubMed Abstract:
Acetyl-CoA carboxylase catalyzes the first committed step in fatty acid synthesis. In Escherichia coli, the enzyme is composed of three distinct protein components: biotin carboxylase, biotin carboxyl carrier protein, and carboxyltransferase. The biotin carboxylase component has served for many years as a paradigm for mechanistic studies devoted toward understanding more complicated biotin-dependent carboxylases. The three-dimensional x-ray structure of an unliganded form of E. coli biotin carboxylase was originally solved in 1994 to 2.4-A resolution. This study revealed the architecture of the enzyme and demonstrated that the protein belongs to the ATP-grasp superfamily. Here we describe the three-dimensional structure of the E. coli biotin carboxylase complexed with ATP and determined to 2.5-A resolution. The major conformational change that occurs upon nucleotide binding is a rotation of approximately 45(o) of one domain relative to the other domains thereby closing off the active site pocket. Key residues involved in binding the nucleotide to the protein include Lys-116, His-236, and Glu-201. The backbone amide groups of Gly-165 and Gly-166 participate in hydrogen bonding interactions with the phosphoryl oxygens of the nucleotide. A comparison of this closed form of biotin carboxylase with carbamoyl-phosphate synthetase is presented.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin, Madison, Wisconsin 53705, USA.