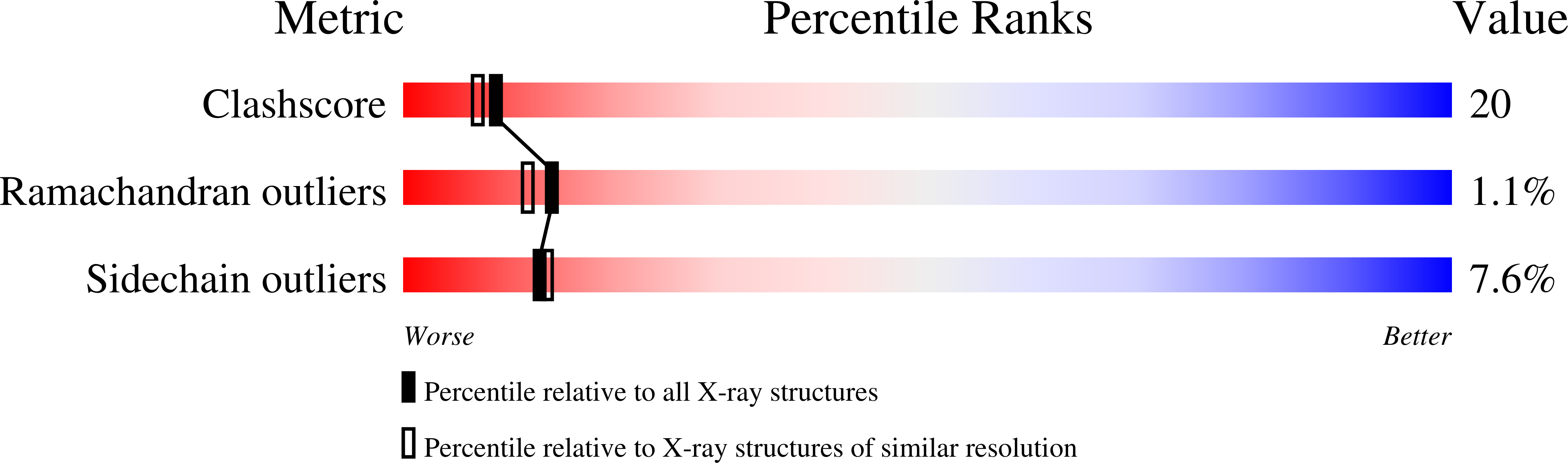Crystal structure of the all-ferrous [4Fe-4S]0 form of the nitrogenase iron protein from Azotobacter vinelandii.
Strop, P., Takahara, P.M., Chiu, H., Angove, H.C., Burgess, B.K., Rees, D.C.(2001) Biochemistry 40: 651-656
- PubMed: 11170381
- DOI: https://doi.org/10.1021/bi0016467
- Primary Citation of Related Structures:
1G1M, 1G5P - PubMed Abstract:
The structure of the nitrogenase iron protein from Azotobacter vinelandii in the all-ferrous [4Fe-4S](0) form has been determined to 2.25 A resolution by using the multiwavelength anomalous diffraction (MAD) phasing technique. The structure demonstrates that major conformational changes are not necessary either in the iron protein or in the cluster to accommodate cluster reduction to the [4Fe-4S](0) oxidation state. A survey of [4Fe-4S] clusters coordinated by four cysteine ligands in proteins of known structure reveals that the [4Fe-4S] cluster of the iron protein has the largest accessible surface area, suggesting that solvent exposure may be relevant to the ability of the iron protein to exist in three oxidation states.
Organizational Affiliation:
Biochemistry Option, California Institute of Technology, Mail Code 147-75, Pasadena, California 91125, USA.