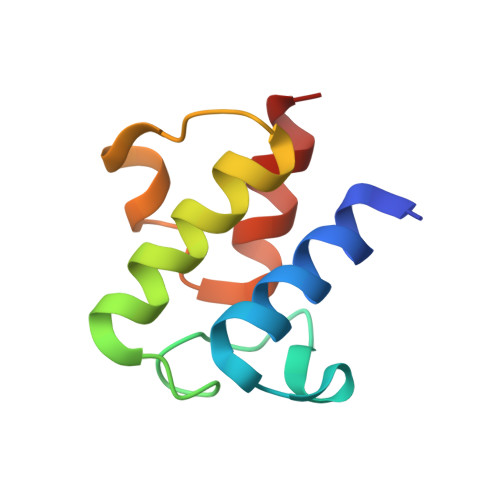
X-ray crystallographic studies on butyryl-ACP reveal flexibility of the structure around a putative acyl chain binding site
Roujeinikova, A., Baldock, C., Simon, W.J., Gilroy, J., Baker, P.J., Stuitje, A.R., Rice, D.W., Slabas, A.R., Rafferty, J.B.(2002) Structure 10: 825-835
- PubMed: 12057197
- DOI: https://doi.org/10.1016/s0969-2126(02)00775-x
- Primary Citation of Related Structures:
1L0H, 1L0I - PubMed Abstract:
Acyl carrier protein (ACP) is an essential cofactor in biosynthesis of fatty acids and many other reactions that require acyl transfer steps. We have determined the first crystal structures of an acylated form of ACP from E. coli, that of butyryl-ACP. Our analysis of the molecular surface of ACP reveals a plastic hydrophobic cavity in the vicinity of the phosphopantethylated Ser36 residue that is expanded and occupied by the butyryl and beta-mercaptoethylamine moieties of the acylated 4'-phosphopantetheine group in one of our crystal forms. In the other form, the cavity is contracted, and we propose that the protein has adopted the conformation after delivery of substrate into the active site of a partner enzyme.
Organizational Affiliation:
Krebs Institute for Biomolecular Research, Department of Molecular Biology and Biotechnology, The University of Sheffield, S10 2TN, Sheffield, United Kingdom.