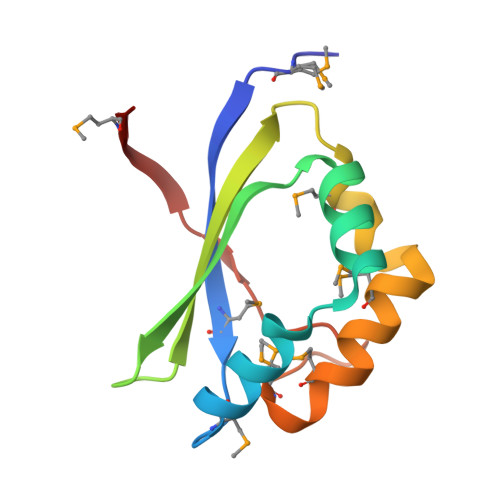
RhaU of Rhizobium leguminosarum is a rhamnose mutarotase.
Richardson, J.S., Carpena, X., Switala, J., Perez-Luque, R., Donald, L.J., Loewen, P.C., Oresnik, I.J.(2008) J Bacteriol 190: 2903-2910
- PubMed: 18156270
- DOI: https://doi.org/10.1128/JB.01120-07
- Primary Citation of Related Structures:
2QLW, 2QLX - PubMed Abstract:
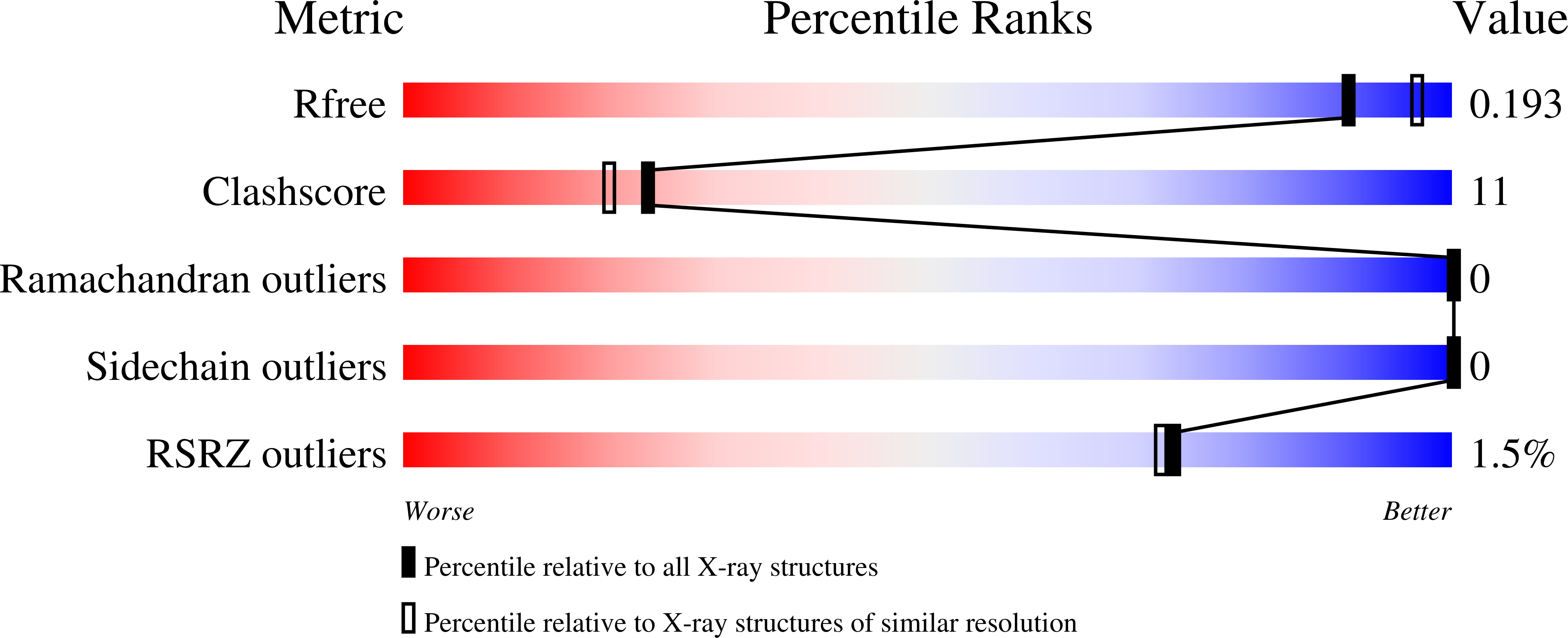
Of the nine genes comprising the L-rhamnose operon of Rhizobium leguminosarum, rhaU has not been assigned a function. The construction of a Delta rhaU strain revealed a growth phenotype that was slower than that of the wild-type strain, although the ultimate cell yields were equivalent. The transport of L-rhamnose into the cell and the rate of its phosphorylation were unaffected by the mutation. RhaU exhibits weak sequence similarity to the formerly hypothetical protein YiiL of Escherichia coli that has recently been characterized as an L-rhamnose mutarotase. To characterize RhaU further, a His-tagged variant of the protein was prepared and subjected to mass spectrometry analysis, confirming the subunit size and demonstrating its dimeric structure. After crystallization, the structure was refined to a 1.6-A resolution to reveal a dimer in the asymmetric unit with a very similar structure to that of YiiL. Soaking a RhaU crystal with L-rhamnose resulted in the appearance of beta-L-rhamnose in the active site.
Organizational Affiliation:
Department of Microbiology, University of Manitoba, Winnipeg, MB R3T 2N2, Canada.