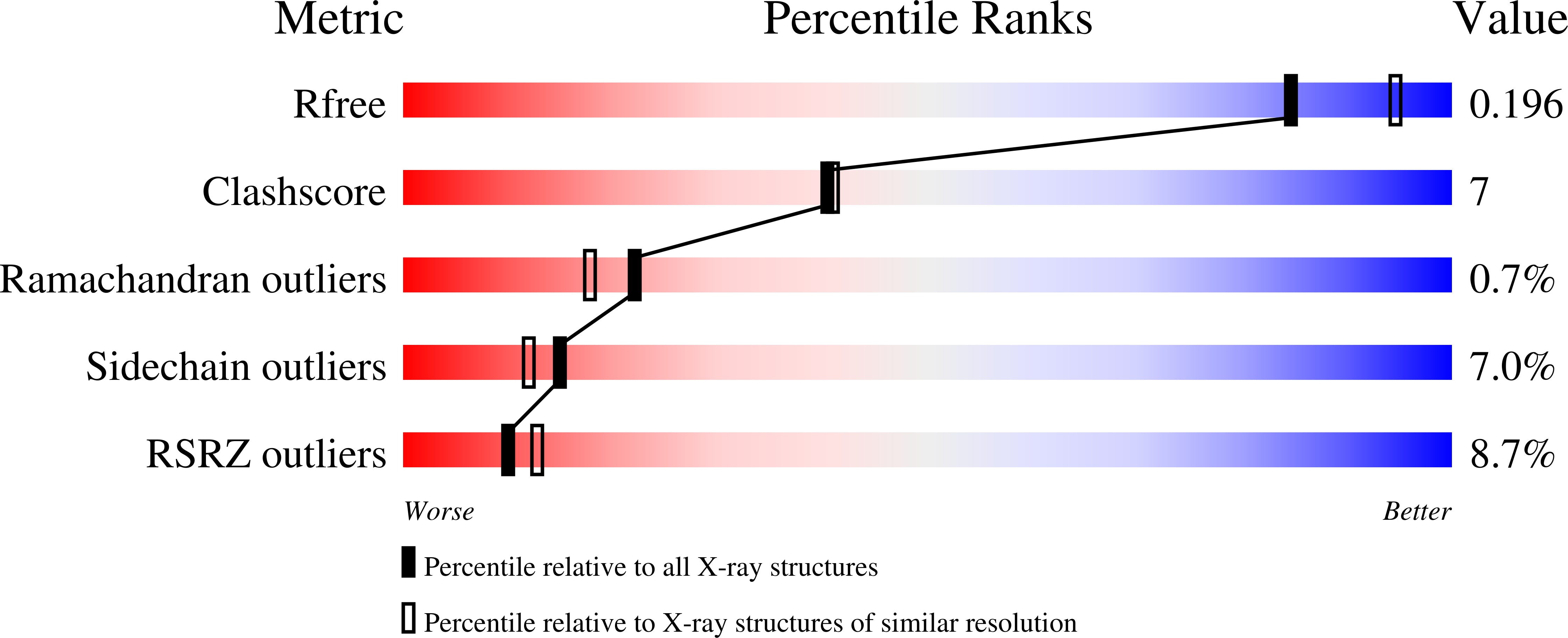
Arginine 104 is a key catalytic residue in leukotriene C4 synthase.
Rinaldo-Matthis, A., Wetterholm, A., Martinez Molina, D., Holm, J., Niegowski, D., Ohlson, E., Nordlund, P., Morgenstern, R., Haeggstrom, J.Z.(2010) J Biol Chem 285: 40771-40776
- PubMed: 20980252
- DOI: https://doi.org/10.1074/jbc.M110.105940
- Primary Citation of Related Structures:
3LEO - PubMed Abstract:
Human leukotriene C(4) synthase (hLTC(4)S) is an integral membrane enzyme that conjugates leukotriene (LT) A(4) with glutathione to form LTC(4), a precursor to the cysteinyl leukotrienes (LTC(4), LTD(4), and LTE(4)) that are involved in the pathogenesis of human bronchial asthma. From the crystal structure of hLTC(4)S, Arg-104 and Arg-31 have been implicated in the conjugation reaction. Here, we used site-directed mutagenesis, UV spectroscopy, and x-ray crystallography to examine the catalytic role of Arg-104 and Arg-31. Exchange of Arg-104 with Ala, Ser, Thr, or Lys abolished 94.3-99.9% of the specific activity against LTA(4). Steady-state kinetics of R104A and R104S revealed that the K(m) for GSH was not significantly affected. UV difference spectra of the binary enzyme-GSH complex indicated that GSH ionization depends on the presence of Arg-104 because no thiolate signal, with λ(max) at 239 nm, could be detected using R104A or R104S hLTC(4)S. Apparently, the interaction of Arg-104 with the thiol group of GSH reduces its pK(a) to allow formation of a thiolate anion and subsequent nucleophilic attack at C6 of LTA(4). On the other hand, exchange of Arg-31 with Ala or Glu reduced the catalytic activity of hLTC(4)S by 88 and 70%, respectively, without significantly affecting the k(cat)/K(m) values for GSH, and a crystal structure of R31Q hLTC(4)S (2.1 Å) revealed a Gln-31 side chain pointing away from the active site. We conclude that Arg-104 plays a critical role in the catalytic mechanism of hLTC(4)S, whereas a functional role of Arg-31 seems more elusive. Because Arg-104 is a conserved residue, our results pertain to other homologous membrane proteins and represent a structure-function paradigm probably common to all microsomal GSH transferases.
Organizational Affiliation:
Division of Chemistry 2, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, S-171 77 Stockholm, Sweden.