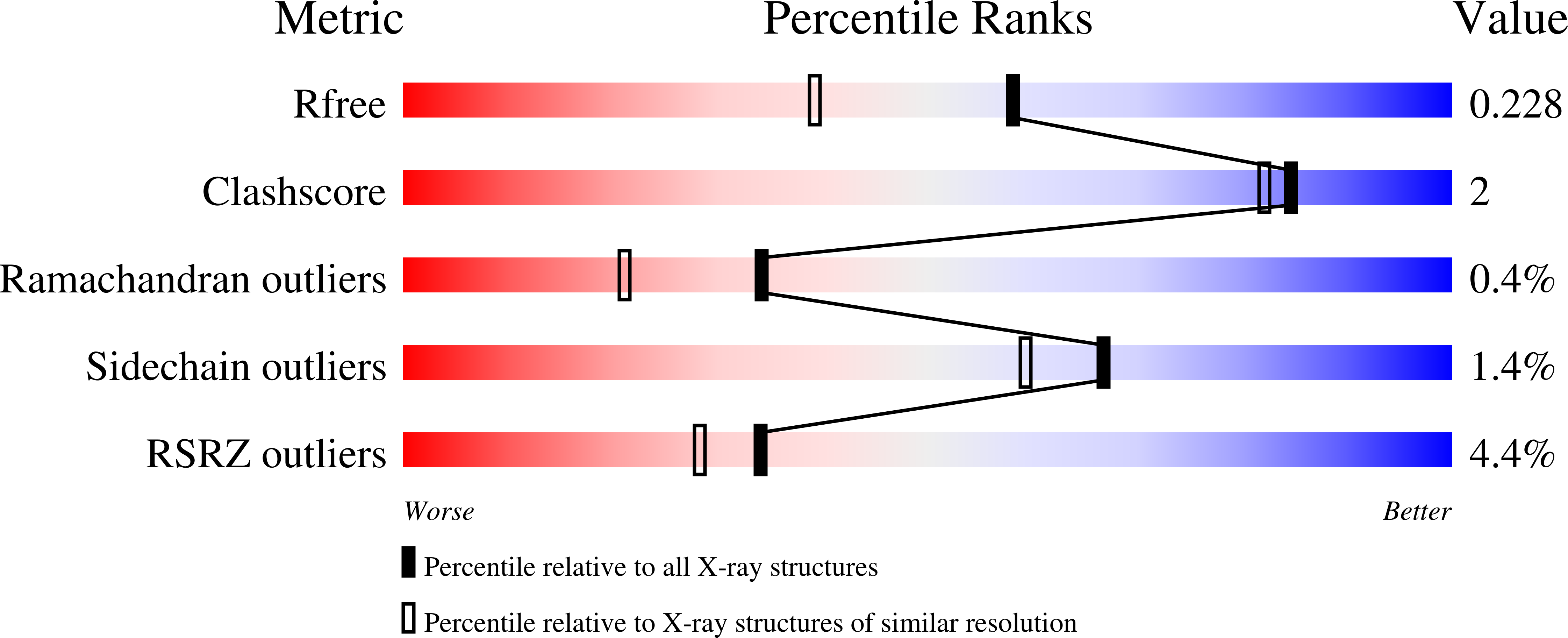
Pseudouridine monophosphate glycosidase: a new glycosidase mechanism.
Huang, S., Mahanta, N., Begley, T.P., Ealick, S.E.(2012) Biochemistry 51: 9245-9255
- PubMed: 23066817
- DOI: https://doi.org/10.1021/bi3006829
- Primary Citation of Related Structures:
4GIJ, 4GIK, 4GIL, 4GIM - PubMed Abstract:
Pseudouridine (Ψ), the most abundant modification in RNA, is synthesized in situ using Ψ synthase. Recently, a pathway for the degradation of Ψ was described [Preumont, A., Snoussi, K., Stroobant, V., Collet, J. F., and Van Schaftingen, E. (2008) J. Biol. Chem. 283, 25238-25246]. In this pathway, Ψ is first converted to Ψ 5'-monophosphate (ΨMP) by Ψ kinase and then ΨMP is degraded by ΨMP glycosidase to uracil and ribose 5-phosphate. ΨMP glycosidase is the first example of a mechanistically characterized enzyme that cleaves a C-C glycosidic bond. Here we report X-ray crystal structures of Escherichia coli ΨMP glycosidase and a complex of the K166A mutant with ΨMP. We also report the structures of a ring-opened ribose 5-phosphate adduct and a ring-opened ribose ΨMP adduct. These structures provide four snapshots along the reaction coordinate. The structural studies suggested that the reaction utilizes a Lys166 adduct during catalysis. Biochemical and mass spectrometry data further confirmed the existence of a lysine adduct. We used site-directed mutagenesis combined with kinetic analysis to identify roles for specific active site residues. Together, these data suggest that ΨMP glycosidase catalyzes the cleavage of the C-C glycosidic bond through a novel ribose ring-opening mechanism.
Organizational Affiliation:
Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, United States.