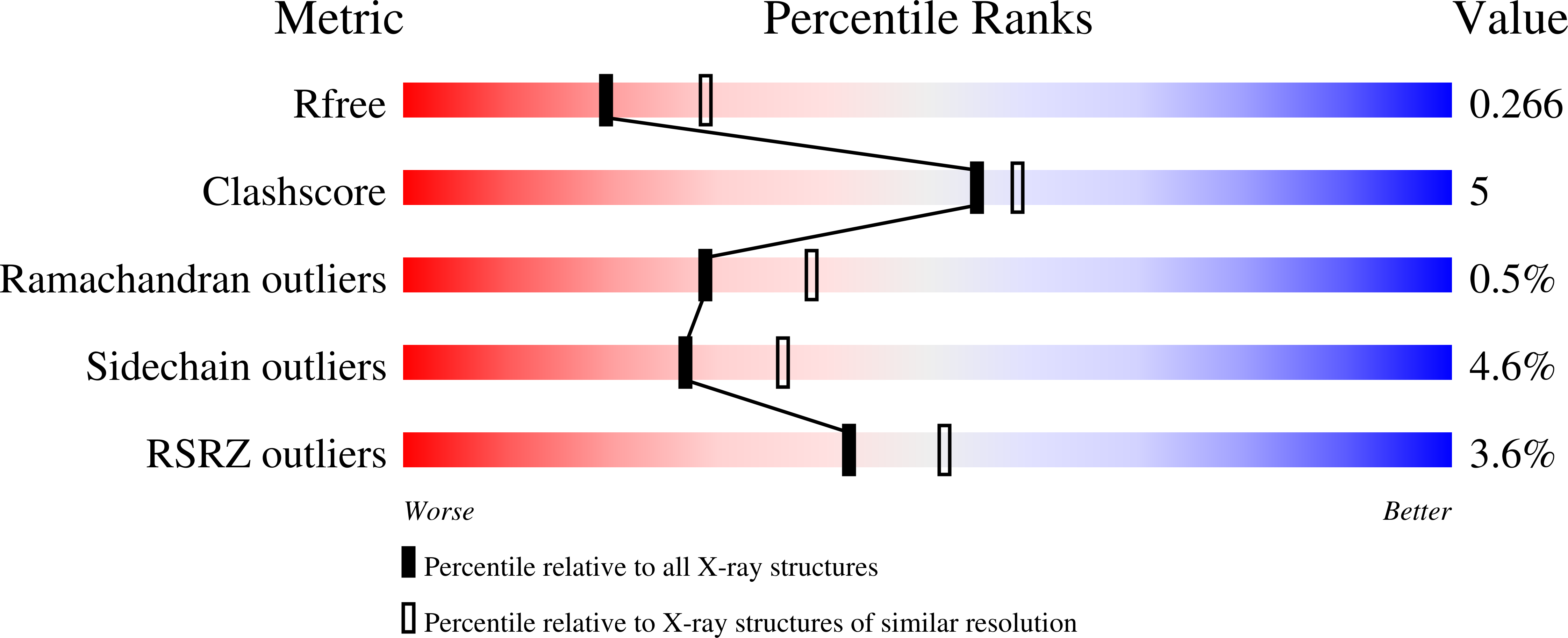
Crystal structure of the ectoine hydroxylase, a snapshot of the active site.
Hoppner, A., Widderich, N., Lenders, M., Bremer, E., Smits, S.H.(2014) J Biol Chem 289: 29570-29583
- PubMed: 25172507
- DOI: https://doi.org/10.1074/jbc.M114.576769
- Primary Citation of Related Structures:
4MHR, 4MHU, 4Q5O - PubMed Abstract:
Ectoine and its derivative 5-hydroxyectoine are compatible solutes that are widely synthesized by bacteria to cope physiologically with osmotic stress. They also serve as chemical chaperones and maintain the functionality of macromolecules. 5-Hydroxyectoine is produced from ectoine through a stereo-specific hydroxylation, an enzymatic reaction catalyzed by the ectoine hydroxylase (EctD). The EctD protein is a member of the non-heme-containing iron(II) and 2-oxoglutarate-dependent dioxygenase superfamily and is evolutionarily well conserved. We studied the ectoine hydroxylase from the cold-adapted marine ultra-microbacterium Sphingopyxis alaskensis (Sa) and found that the purified SaEctD protein is a homodimer in solution. We determined the SaEctD crystal structure in its apo-form, complexed with the iron catalyst, and in a form that contained iron, the co-substrate 2-oxoglutarate, and the reaction product of EctD, 5-hydroxyectoine. The iron and 2-oxoglutarate ligands are bound within the EctD active site in a fashion similar to that found in other members of the dioxygenase superfamily. 5-Hydroxyectoine, however, is coordinated by EctD in manner different from that found in high affinity solute receptor proteins operating in conjunction with microbial import systems for ectoines. Our crystallographic analysis provides a detailed view into the active site of the ectoine hydroxylase and exposes an intricate network of interactions between the enzyme and its ligands that collectively ensure the hydroxylation of the ectoine substrate in a position- and stereo-specific manner.
Organizational Affiliation:
From the X-ray Facility and Crystal Farm, Heinrich-Heine-University at Düsseldorf, D-40225 Düsseldorf.