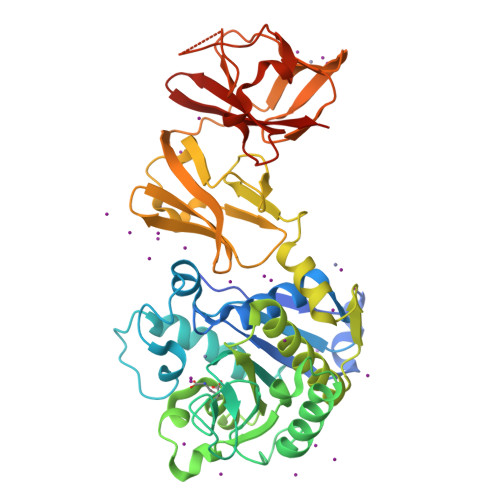
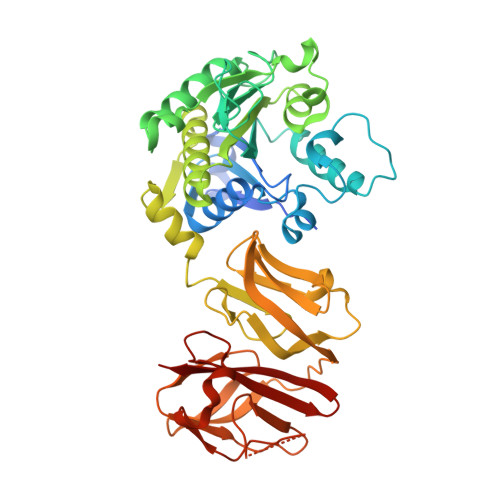
Helical Shape of Helicobacter pylori Requires an Atypical Glutamine as a Zinc Ligand in the Carboxypeptidase Csd4.
Chan, A.C., Blair, K.M., Liu, Y., Frirdich, E., Gaynor, E.C., Tanner, M.E., Salama, N.R., Murphy, M.E.(2015) J Biological Chem 290: 3622-3638
- PubMed: 25505267
- DOI: https://doi.org/10.1074/jbc.M114.624734
- Primary Citation of Related Structures:
4WCK, 4WCL, 4WCM, 4WCN - PubMed Abstract:
Peptidoglycan modifying carboxypeptidases (CPs) are important determinants of bacterial cell shape. Here, we report crystal structures of Csd4, a three-domain protein from the human gastric pathogen Helicobacter pylori. The catalytic zinc in Csd4 is coordinated by a rare His-Glu-Gln configuration that is conserved among most Csd4 homologs, which form a distinct subfamily of CPs. Substitution of the glutamine to histidine, the residue found in prototypical zinc carboxypeptidases, resulted in decreased enzyme activity and inhibition by phosphate. Expression of the histidine variant at the native locus in a H. pylori csd4 deletion strain did not restore the wild-type helical morphology. Biochemical assays show that Csd4 can cleave a tripeptide peptidoglycan substrate analog to release m-DAP. Structures of Csd4 with this substrate analog or product bound at the active site reveal determinants of peptidoglycan specificity and the mechanism to cleave an isopeptide bond to release m-DAP. Our data suggest that Csd4 is the archetype of a new CP subfamily with a domain scheme that differs from this large family of peptide-cleaving enzymes.
Organizational Affiliation:
From the Department of Microbiology and Immunology, University of British Columbia, Vancouver, British Columbia V6T 1Z3, Canada.