Disentangling the formation, mechanism, and evolvement of the covalent methanesulfonyl fluoride acetylcholinesterase adduct: Insights into an aged-like inactive complex susceptible to reactivation by a combination of nucleophiles.
Stojan, J., Pesaresi, A., Meden, A., Lamba, D.(2024) Protein Sci 33: e4977-e4977
- PubMed: 38591646
- DOI: https://doi.org/10.1002/pro.4977
- Primary Citation of Related Structures:
5EHX, 5EI5 - PubMed Abstract:
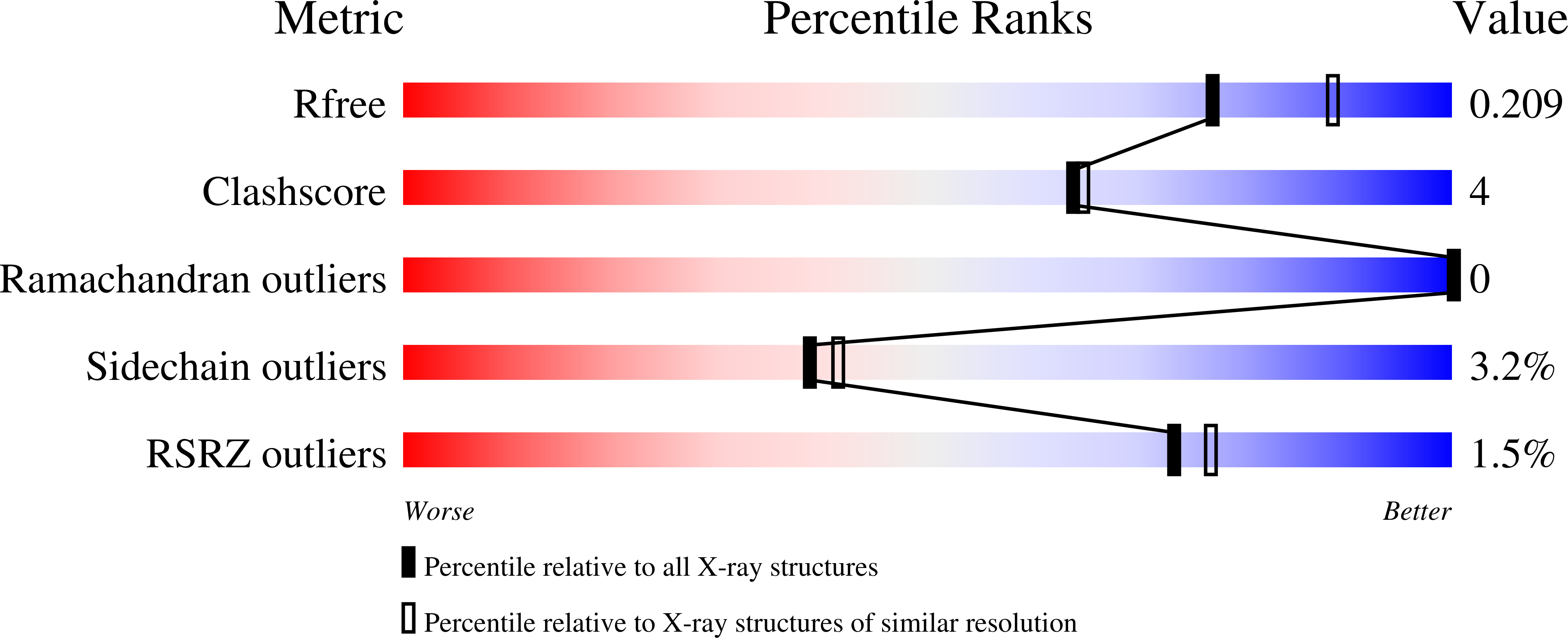
Chemical warfare nerve agents and pesticides, known as organophosphorus compounds inactivate cholinesterases (ChEs) by phosphorylating the serine hydroxyl group located at the active site of ChEs. Over the course of time, phosphorylation is followed by loss of an organophosphate-leaving group and the bond with ChEs becomes irreversible, a process known as aging. Differently, structurally related irreversible catalytic poisons bearing sulfur instead of phosphorus convert ChEs in its aged form only by covalently binding to the key catalytic serine. Kinetic and crystallographic studies of the interaction between Torpedo californica acetylcholinesterase (TcAChE) and a small organosulfonate, methanesulfonyl fluoride (MSF), indeed revealed irreversibly methylsulfonylated serine 200, to be isosteric with the bound aged sarin/soman analogues. The potent bulky reversible inhibitor 7-bis-tacrine (BTA) adopts, in the active site of the crystal structure of the MSF-enzyme adduct, a location and an orientation that closely resemble the one being found in the crystal structure of the BTA-enzyme complex. Remarkably, the presence of BTA accelerates the rate of methanesulfonylation by a factor of two. This unexpected result can be explained on the basis of two facts: i) the steric hindrance exerted by BTA to MSF in accessing the active site and ii) the acceleration of the MSF-enzyme adduct formation as a consequence of the lowering of the rotational and translational degrees of freedom in the proximity of the catalytic serine. It is well known that pralidoxime (2-Pyridine Aldoxime Methyl chloride, 2-PAM) alone or in the presence of the substrate acetylcholine cannot reactivate the active site serine of the TcAChE-MSF adduct. We show that the simultaneous presence of 2-PAM and the additional neutral oxime, 2-[(hydroxyimino)methyl]-l-methylimidazol (2-HAM), triggers the reactivation process of TcAChE within the hour timescale. Overall, our results pave the way toward the likely use of a cocktail of distinctive oximes as a promising recipe for an effective and fast reactivation of aged cholinesterases.
Organizational Affiliation:
Institute of Biochemistry, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia.