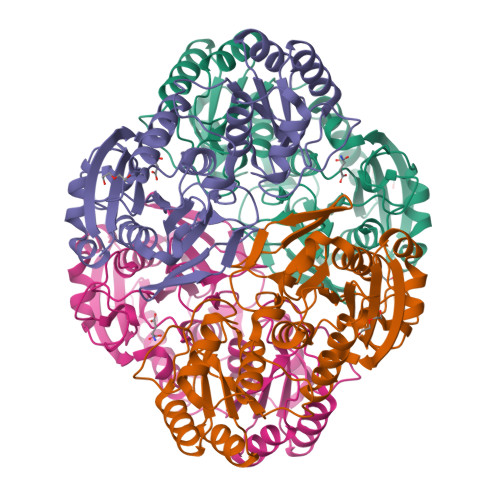
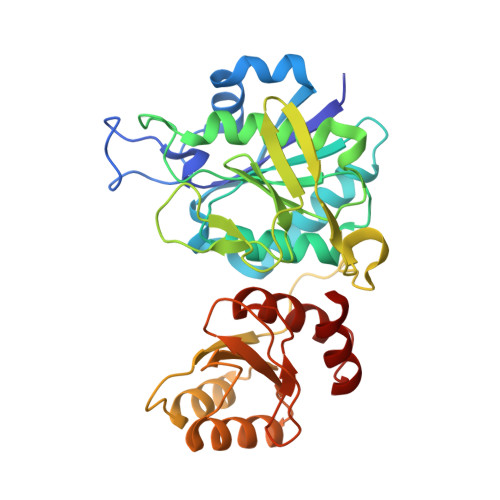
Structural Insight into Substrate Selectivity of Erwinia chrysanthemi l-Asparaginase.
Nguyen, H.A., Su, Y., Lavie, A.(2016) Biochemistry 55: 1246-1253
- PubMed: 26855287
- DOI: https://doi.org/10.1021/acs.biochem.5b01351
- Primary Citation of Related Structures:
5F52, 5HW0 - PubMed Abstract:
l-Asparaginases of bacterial origin are a mainstay of acute lymphoblastic leukemia treatment. The mechanism of action of these enzyme drugs is associated with their capacity to deplete the amino acid l-asparagine from the blood. However, clinical use of bacterial l-asparaginases is complicated by their dual l-asparaginase and l-glutaminase activities. The latter, even though representing only ∼10% of the overall activity, is partially responsible for the observed toxic side effects. Hence, l-asparaginases devoid of l-glutaminase activity hold potential as safer drugs. Understanding the key determinants of l-asparaginase substrate specificity is a prerequisite step toward the development of enzyme variants with reduced toxicity. Here we present crystal structures of the Erwinia chrysanthemi l-asparaginase in complex with l-aspartic acid and with l-glutamic acid. These structures reveal two enzyme conformations-open and closed-corresponding to the inactive and active states, respectively. The binding of ligands induces the positioning of the catalytic Thr15 into its active conformation, which in turn allows for the ordering and closure of the flexible N-terminal loop. Notably, l-aspartic acid is more efficient than l-glutamic acid in inducing the active positioning of Thr15. Structural elements explaining the preference of the enzyme for l-asparagine over l-glutamine are discussed with guidance to the future development of more specific l-asparaginases.
Organizational Affiliation:
The Jesse Brown VA Medical Center , Chicago, Illinois 60607, United States.