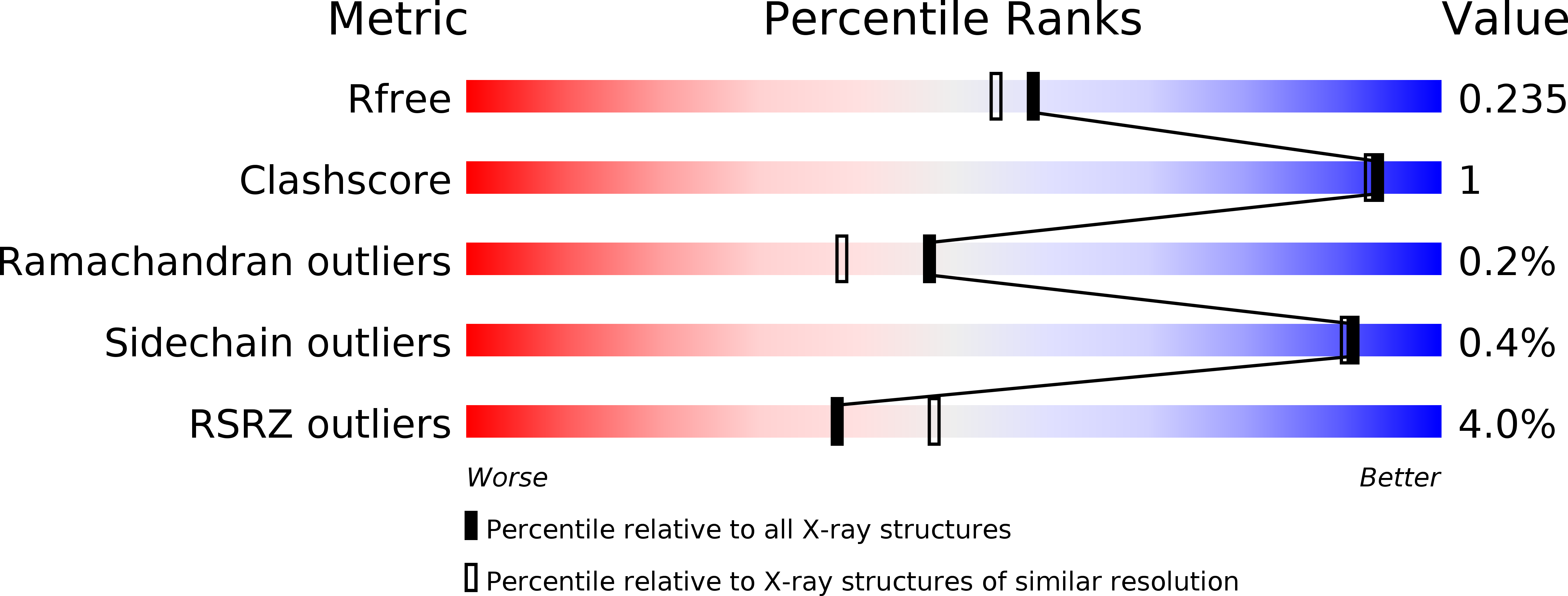
Optimisation of a novel series of potent and orally bioavailable azanaphthyridine SYK inhibitors.
Garton, N.S., Barker, M.D., Davis, R.P., Douault, C., Hooper-Greenhill, E., Jones, E., Lewis, H.D., Liddle, J., Lugo, D., McCleary, S., Preston, A.G., Ramirez-Molina, C., Neu, M., Shipley, T.J., Somers, D.O., Watson, R.J., Wilson, D.M.(2016) Bioorg Med Chem Lett 26: 4606-4612
- PubMed: 27578246
- DOI: https://doi.org/10.1016/j.bmcl.2016.08.070
- Primary Citation of Related Structures:
5LMA, 5LMB - PubMed Abstract:
The optimisation of the azanaphthyridine series of Spleen Tyrosine Kinase inhibitors is described. The medicinal chemistry strategy was focused on optimising the human whole blood activity whilst achieving a sufficient margin over hERG activity. A good pharmacokinetic profile was achieved by modification of the pKa. Morpholine compound 32 is a potent SYK inhibitor showing moderate selectivity, good oral bioavailability and good efficacy in the rat Arthus model but demonstrated a genotoxic potential in the Ames assay.
Organizational Affiliation:
GlaxoSmithKline R&D, Medicines Research Centre, Gunnels Wood Road, Stevenage, Hertfordshire, SG1 2NY, UK. Electronic address: Neil.S.Garton@gsk.com.