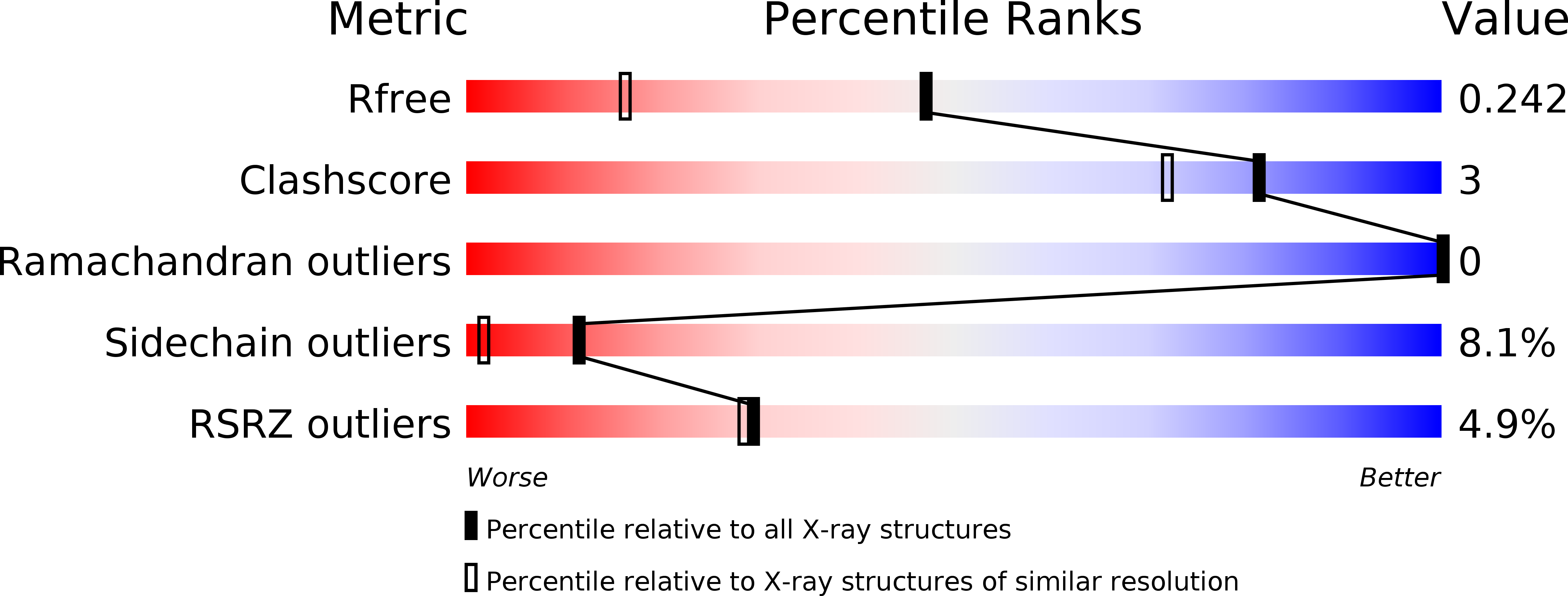
PanDDA analysis group deposition of models with modelled events (e.g. bound ligands)
Pinkas, D.M., Bufton, J.C., Fox, A.E., Talon, R., Krojer, T., Douangamath, A., Collins, P., Zhang, R., von Delft, F., Bountra, C., Arrowsmith, C.H., Edwards, A., Bullock, A.N.To be published.