Molecular Basis for Olefin Rearrangement in the Gephyronic Acid Polyketide Synthase.
Dodge, G.J., Ronnow, D., Taylor, R.E., Smith, J.L.(2018) ACS Chem Biol 13: 2699-2707
- PubMed: 30179448
- DOI: https://doi.org/10.1021/acschembio.8b00645
- Primary Citation of Related Structures:
6MBF, 6MBG, 6MBH - PubMed Abstract:
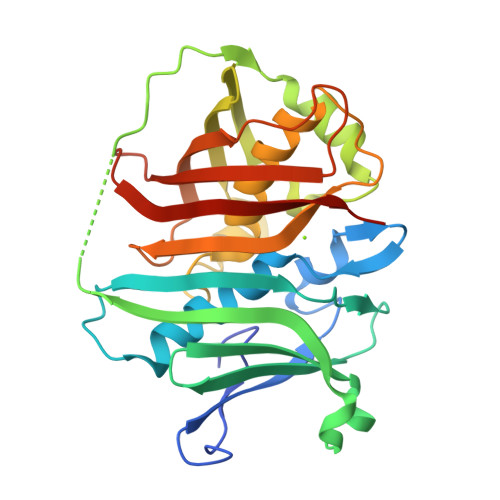
Polyketide synthases (PKS) are a rich source of natural products of varied chemical composition and biological significance. Here, we report the characterization of an atypical dehydratase (DH) domain from the PKS pathway for gephyronic acid, an inhibitor of eukaryotic protein synthesis. Using a library of synthetic substrate mimics, the reaction course, stereospecificity, and tolerance to non-native substrates of GphF DH1 are probed via LC-MS analysis. Taken together, the studies establish GphF DH1 as a dual-function dehydratase/isomerase that installs an odd-to-even double bond and yields a product consistent with the isobutenyl terminus of gephyronic acid. The studies also reveal an unexpected C2 epimerase function in catalytic turnover with the native substrate. A 1.55-Å crystal structure of GphF DH1 guided mutagenesis experiments to elucidate the roles of key amino acids in the multistep DH1 catalysis, identifying critical functions for leucine and tyrosine side chains. The mutagenesis results were applied to add a secondary isomerase functionality to a nonisomerizing DH in the first successful gain-of-function engineering of a PKS DH. Our studies of GphF DH1 catalysis highlight the versatility of the DH active site and adaptation for a specific catalytic outcome with a specific substrate.
Organizational Affiliation:
Department of Biological Chemistry and Life Sciences Institute , University of Michigan , Ann Arbor , Michigan 48109 , United States.