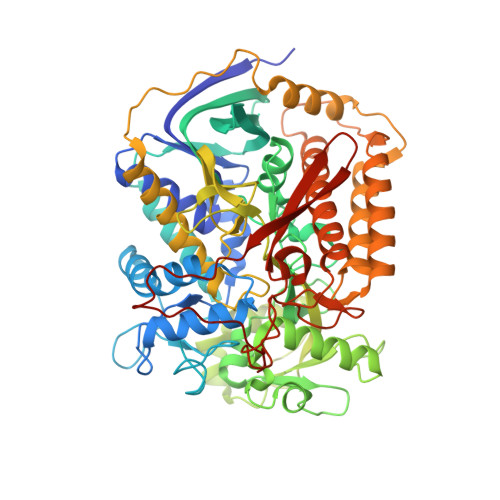
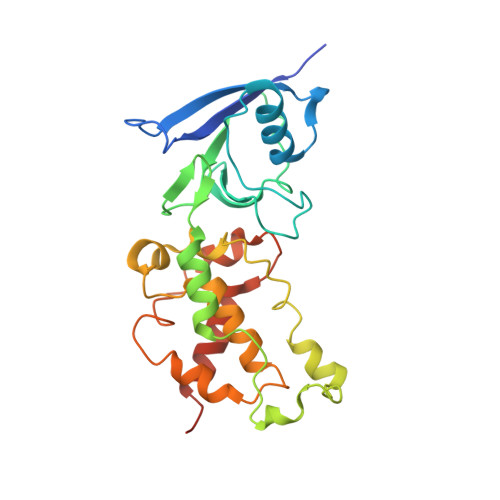
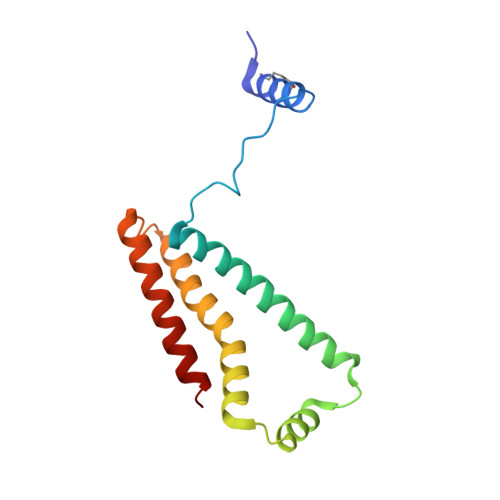
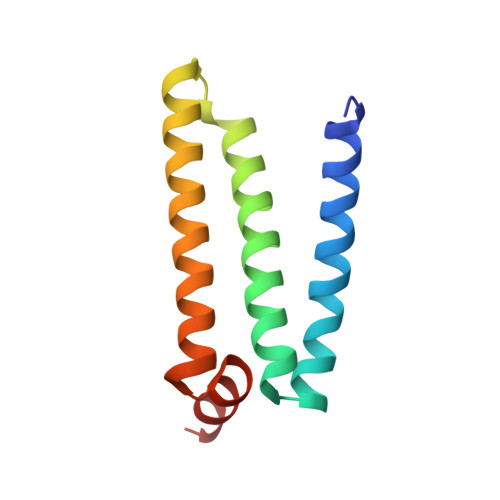
Crystallographic investigation of the ubiquinone binding site of respiratory Complex II and its inhibitors.
Huang, L.S., Lummen, P., Berry, E.A.(2021) Biochim Biophys Acta Proteins Proteom 1869: 140679-140679
- PubMed: 34089891
- DOI: https://doi.org/10.1016/j.bbapap.2021.140679
- Primary Citation of Related Structures:
6MYO, 6MYP, 6MYQ, 6MYR, 6MYS, 6MYT, 6MYU - PubMed Abstract:
The quinone binding site (Q-site) of Mitochondrial Complex II (succinate-ubiquinone oxidoreductase) is the target for a number of inhibitors useful for elucidating the mechanism of the enzyme. Some of these have been developed as fungicides or pesticides, and species-specific Q-site inhibitors may be useful against human pathogens. We report structures of chicken Complex II with six different Q-site inhibitors bound, at resolutions 2.0-2.4 Å. These structures show the common interactions between the inhibitors and their binding site. In every case a carbonyl or hydroxyl oxygen of the inhibitor is H-bonded to Tyr58 in subunit SdhD and Trp173 in subunit SdhB. Two of the inhibitors H-bond Ser39 in subunit SdhC directly, while two others do so via a water molecule. There is a distinct cavity that accepts the 2-substituent of the carboxylate ring in flutolanil and related inhibitors. A hydrophobic "tail pocket" opens to receive a side-chain of intermediate-length inhibitors. Shorter inhibitors fit entirely within the main binding cleft, while the long hydrophobic side chains of ferulenol and atpenin A5 protrude out of the cleft into the bulk lipid region, as presumably does that of ubiquinone. Comparison of mitochondrial and Escherichia coli Complex II shows a rotation of the membrane-anchor subunits by 7° relative to the iron‑sulfur protein. This rotation alters the geometry of the Q-site and the H-bonding pattern of SdhB:His216 and SdhD:Asp57. This conformational difference, rather than any active-site mutation, may be responsible for the different inhibitor sensitivity of the bacterial enzyme.
Organizational Affiliation:
Biochemistry and Molecular Biology, SUNY Upstate Medical University, 750 E. Adams Street, Syracuse, N.Y 13210, USA.