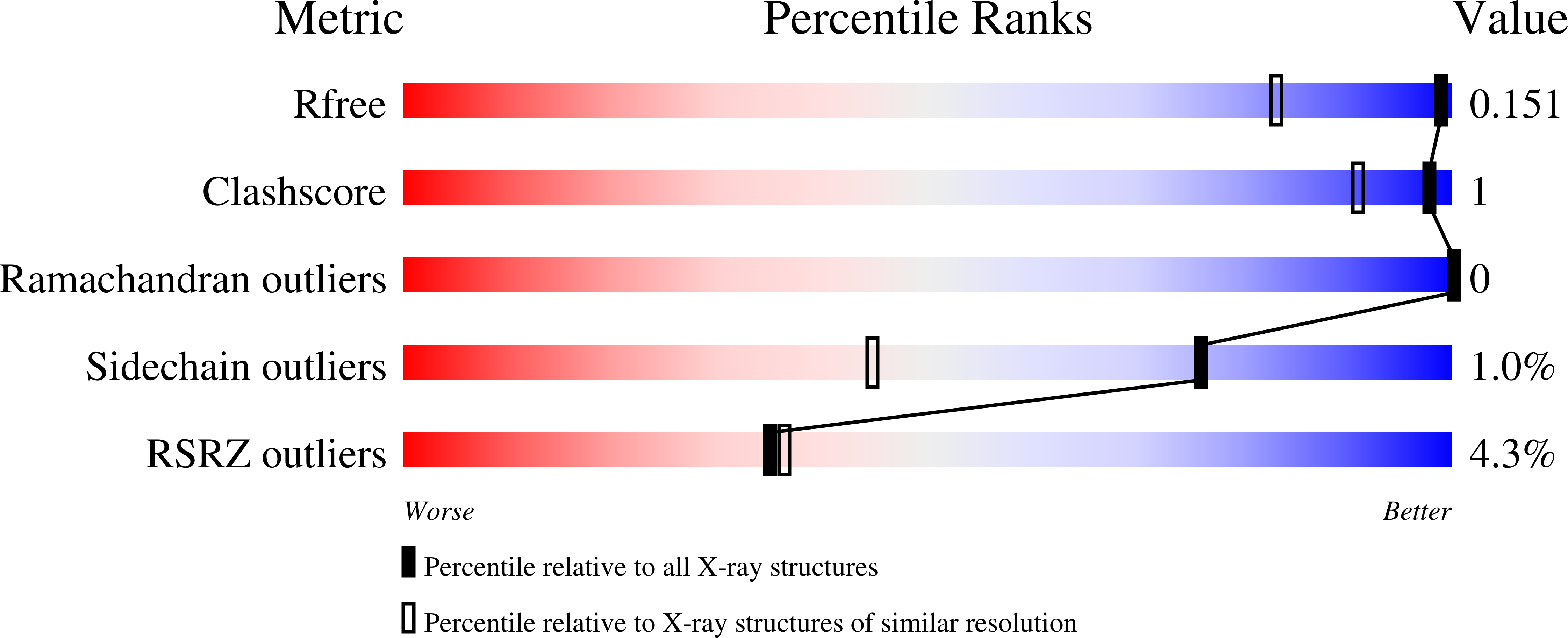
The Oxoglutarate Binding Site and Regulatory Mechanism Are Conserved in Ammonium Transporter Inhibitors GlnKs from Methanococcales .
Muller, M.C., Wagner, T.(2021) Int J Mol Sci 22
- PubMed: 34445335
- DOI: https://doi.org/10.3390/ijms22168631
- Primary Citation of Related Structures:
7P4V, 7P4Y, 7P50, 7P52 - PubMed Abstract:
Protein inhibition is a natural regulatory process to control cellular metabolic fluxes. P II -family signal-transducing effectors are in this matter key regulators of the nitrogen metabolism. Their interaction with their various targets is governed by the cellular nitrogen level and the energy charge. Structural studies on GlnK, a P II -family inhibitor of the ammonium transporters (Amt), showed that the T-loops responsible for channel obstruction are displaced upon the binding of 2-oxoglutarate, magnesium and ATP in a conserved cleft. However, GlnK from Methanocaldococcus jannaschii was shown to bind 2-oxoglutarate on the tip of its T-loop, causing a moderate disruption to GlnK-Amt interaction, raising the question if methanogenic archaea use a singular adaptive strategy. Here we show that membrane fractions of Methanothermococcus thermolithotrophicus released GlnKs only in the presence of Mg-ATP and 2-oxoglutarate. This observation led us to structurally characterize the two GlnK isoforms apo or in complex with ligands. Together, our results show that the 2-oxoglutarate binding interface is conserved in GlnKs from Methanococcales , including Methanocaldococcus jannaschii , emphasizing the importance of a free carboxy-terminal group to facilitate ligand binding and to provoke the shift of the T-loop positions.
Organizational Affiliation:
Microbial Metabolism Research Group, Max Planck Institute for Marine Microbiology, Celsiusstraße 1, 28359 Bremen, Germany.