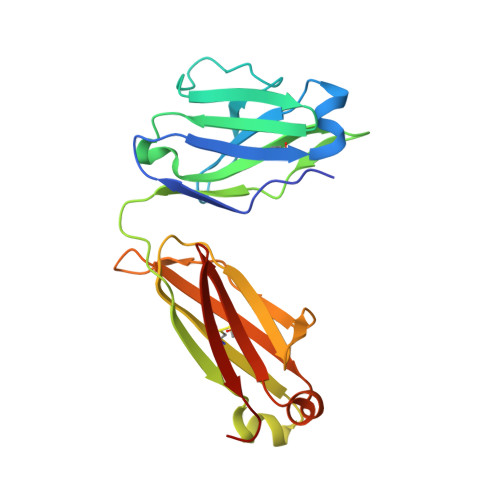
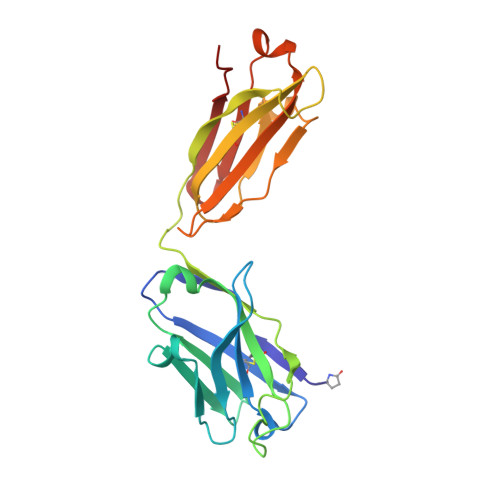
Identification of IOMA-class neutralizing antibodies targeting the CD4-binding site on the HIV-1 envelope glycoprotein.
van Schooten, J., Farokhi, E., Schorcht, A., van den Kerkhof, T.L.G.M., Gao, H., van der Woude, P., Burger, J.A., Meesters, T.G.R., Bijl, T., Ghalaiyini, R., Turner, H.L., Dorning, J., van Schaik, B.D.C., van Kampen, A.H.C., Labranche, C.C., Stanfield, R.L., Sok, D., Montefiori, D.C., Burton, D.R., Seaman, M.S., Ozorowski, G., Wilson, I.A., Sanders, R.W., Ward, A.B., van Gils, M.J.(2022) Nat Commun 13: 4515-4515
- PubMed: 35922441
- DOI: https://doi.org/10.1038/s41467-022-32208-0
- Primary Citation of Related Structures:
7U04, 7U0K, 7Z3A - PubMed Abstract:
A major goal of current HIV-1 vaccine design efforts is to induce broadly neutralizing antibodies (bNAbs). The VH1-2-derived bNAb IOMA directed to the CD4-binding site of the HIV-1 envelope glycoprotein is of interest because, unlike the better-known VH1-2-derived VRC01-class bNAbs, it does not require a rare short light chain complementarity-determining region 3 (CDRL3). Here, we describe three IOMA-class NAbs, ACS101-103, with up to 37% breadth, that share many characteristics with IOMA, including an average-length CDRL3. Cryo-electron microscopy revealed that ACS101 shares interactions with those observed with other VH1-2 and VH1-46-class bNAbs, but exhibits a unique binding mode to residues in loop D. Analysis of longitudinal sequences from the patient suggests that a transmitter/founder-virus lacking the N276 glycan might have initiated the development of these NAbs. Together these data strengthen the rationale for germline-targeting vaccination strategies to induce IOMA-class bNAbs and provide a wealth of sequence and structural information to support such strategies.
Organizational Affiliation:
Department of Medical Microbiology, Amsterdam Infection & Immunity Institute, Amsterdam UMC, Location AMC, University of Amsterdam, Amsterdam, 1105AZ, The Netherlands.