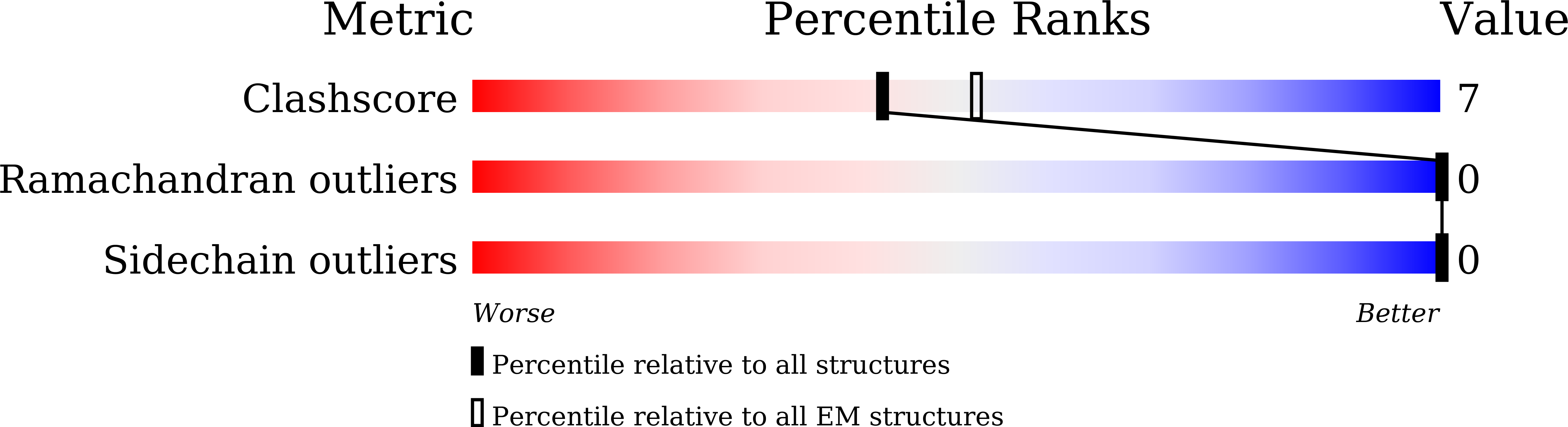Rift Valley fever virus coordinates the assembly of a programmable E3 ligase to promote viral replication.
Li, H., Zhang, Y., Rao, G., Zhang, C., Guan, Z., Huang, Z., Li, S., Lozach, P.Y., Cao, S., Peng, K.(2024) Cell 187: 6896
- PubMed: 39366381
- DOI: https://doi.org/10.1016/j.cell.2024.09.008
- Primary Citation of Related Structures:
8WCM - PubMed Abstract:
Viruses encode strategies to degrade cellular proteins to promote infection and pathogenesis. Here, we revealed that the non-structural protein NSs of Rift Valley fever virus forms a filamentous E3 ligase to trigger efficient degradation of targeted proteins. Reconstitution in vitro and cryoelectron microscopy analysis with the 2.9-Å resolution revealed that NSs forms right-handed helical fibrils. The NSs filamentous oligomers associate with the cellular FBXO3 to form a remodeled E3 ligase. The NSs-FBXO3 E3 ligase targets the cellular TFIIH complex through the NSs-P62 interaction, leading to ubiquitination and proteasome-dependent degradation of the TFIIH complex. NSs-FBXO3-triggered TFIIH complex degradation resulted in robust inhibition of antiviral immunity and promoted viral pathogenesis in vivo. Furthermore, it is demonstrated that NSs can be programmed to target additional proteins for proteasome-dependent degradation, serving as a versatile targeted protein degrader. These results showed that a virulence factor forms a filamentous and programmable degradation machinery to induce organized degradation of cellular proteins to promote viral infection.
Organizational Affiliation:
State Key Laboratory of Virology, Wuhan Institute of Virology, Center for Antiviral Research, Chinese Academy of Sciences, Wuhan 430071, People's Republic of China; University of Chinese Academy of Sciences, Beijing 100049, People's Republic of China.