Structural Insights and Catalytic Mechanism of 3-Hydroxybutyryl-CoA Dehydrogenase from Faecalibacterium Prausnitzii A2-165.
Yang, J., Jeon, H.J., Park, S., Park, J., Jang, S., Shin, B., Bang, K., Hawkes, H.K., Park, S., Kim, S., Hwang, K.Y.(2024) Int J Mol Sci 25
- PubMed: 39409040
- DOI: https://doi.org/10.3390/ijms251910711
- Primary Citation of Related Structures:
9JHE, 9JHY, 9JHZ, 9JI0 - PubMed Abstract:
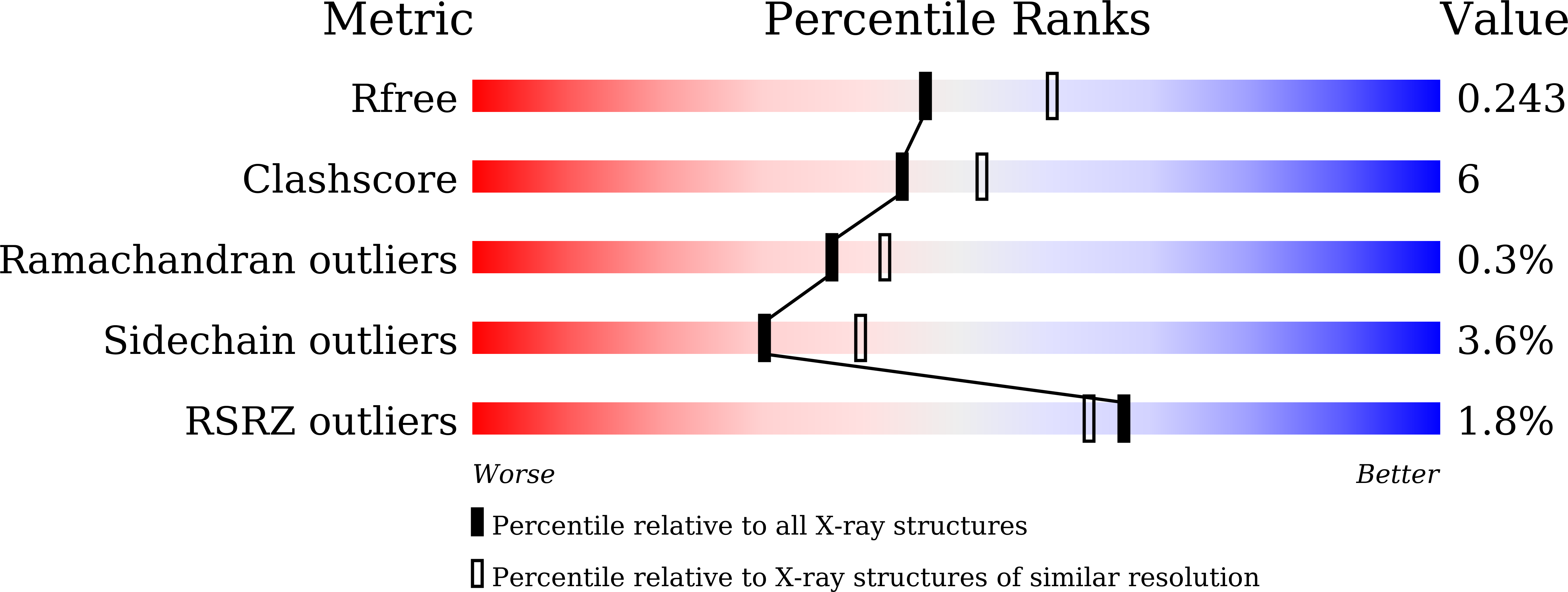
Atopic dermatitis (AD) is characterized by a T-helper cell type 2 (Th2) inflammatory response leading to skin damage with erythema and edema. Comparative fecal sample analysis has uncovered a strong correlation between AD and Faecalibacterium prausnitzii strain A2-165, specifically associated with butyrate production. Therefore, understanding the functional mechanisms of crucial enzymes in the butyrate pathway, such as 3-hydroxybutyryl-CoA dehydrogenase of A2-165 (A2HBD), is imperative. Here, we have successfully elucidated the three-dimensional structure of A2HBD in complex with acetoacetyl-CoA and NAD + at a resolution of 2.2Å using the PAL-11C beamline (third generation). Additionally, X-ray data of A2HBD in complex with acetoacetyl-CoA at a resolution of 1.9 Å were collected at PAL-XFEL (fourth generation) utilizing Serial Femtosecond Crystallography (SFX). The monomeric structure of A2HBD consists of two domains, N-terminal and C-terminal, with cofactor binding occurring at the N-terminal domain, while the C-terminal domain facilitates dimerization. Our findings elucidate the binding mode of NAD + to A2HBD. Upon acetoacetyl-CoA binding, the crystal structure revealed a significant conformational change in the Clamp-roof domain (root-mean-square deviation of 2.202 Å). Notably, residue R143 plays a critical role in capturing the adenine phosphate ring, underlining its significance in substrate recognition and catalytic activity. The binding mode of acetoacetyl-CoA was also clarified, indicating its lower stability compared to NAD + . Furthermore, the conformational change of hydrophobic residues near the catalytic cavity upon substrate binding resulted in cavity shrinkage from an open to closed conformation. This study confirms the conformational changes of catalytic triads involved in the catalytic reaction and presents a proposed mechanism for substrate reduction based on structural observations.
Organizational Affiliation:
Department of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul 02841, Republic of Korea.