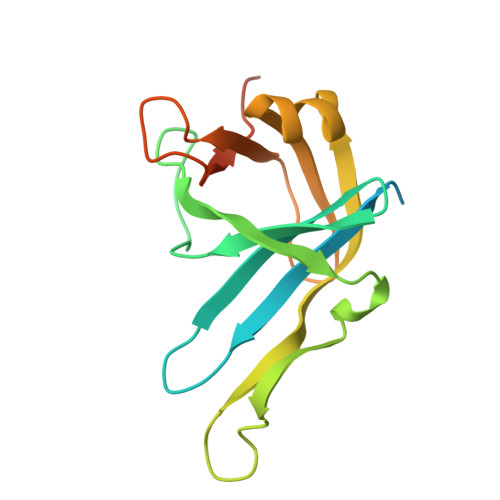
Solution structure and DNA-binding properties of the phosphoesterase domain of DNA ligase D.
Natarajan, A., Dutta, K., Temel, D.B., Nair, P.A., Shuman, S., Ghose, R.(2012) Nucleic Acids Res 40: 2076-2088
- PubMed: 22084199
- DOI: https://doi.org/10.1093/nar/gkr950
- Primary Citation of Related Structures:
2LJ6 - PubMed Abstract:
The phosphoesterase (PE) domain of the bacterial DNA repair enzyme LigD possesses distinctive manganese-dependent 3'-phosphomonoesterase and 3'-phosphodiesterase activities. PE exemplifies a new family of DNA end-healing enzymes found in all phylogenetic domains. Here, we determined the structure of the PE domain of Pseudomonas aeruginosa LigD (PaePE) using solution NMR methodology. PaePE has a disordered N-terminus and a well-folded core that differs in instructive ways from the crystal structure of a PaePE•Mn(2+)• sulfate complex, especially at the active site that is found to be conformationally dynamic. Chemical shift perturbations in the presence of primer-template duplexes with 3'-deoxynucleotide, 3'-deoxynucleotide 3'-phosphate, or 3' ribonucleotide termini reveal the surface used by PaePE to bind substrate DNA and suggest a more efficient engagement in the presence of a 3'-ribonucleotide. Spectral perturbations measured in the presence of weakly catalytic (Cd(2+)) and inhibitory (Zn(2+)) metals provide evidence for significant conformational changes at and near the active site, compared to the relatively modest changes elicited by Mn(2+).
Organizational Affiliation:
Department of Chemistry, The City College of New York, 160 Convent Avenue, New York, NY 10031, USA.