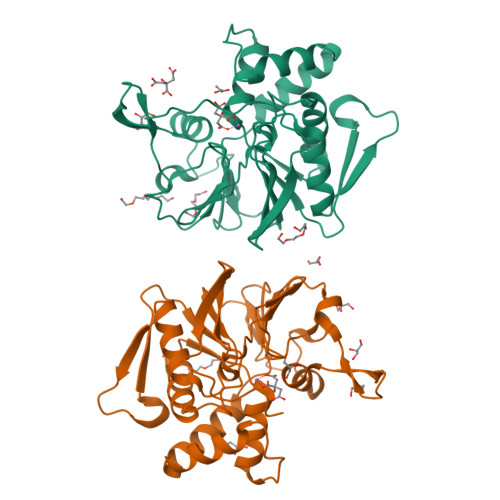
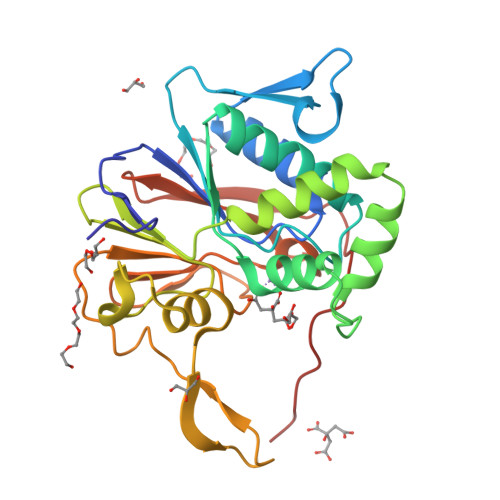
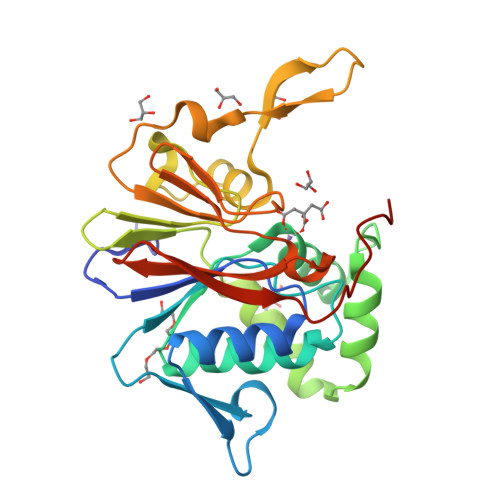
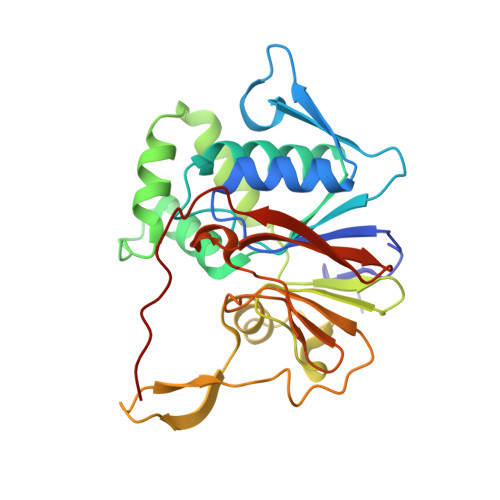
Structure and mechanism of the 2',3' phosphatase component of the bacterial Pnkp-Hen1 RNA repair system.
Wang, L.K., Smith, P., Shuman, S.(2013) Nucleic Acids Res 41: 5864-5873
- PubMed: 23595150
- DOI: https://doi.org/10.1093/nar/gkt221
- Primary Citation of Related Structures:
4J6O - PubMed Abstract:
Pnkp is the end-healing and end-sealing component of an RNA repair system present in diverse bacteria from many phyla. Pnkp is composed of three catalytic modules: an N-terminal polynucleotide 5' kinase, a central 2',3' phosphatase and a C-terminal ligase. The phosphatase module is a Mn(2+)-dependent phosphodiesterase-monoesterase that dephosphorylates 2',3'-cyclic phosphate RNA ends. Here we report the crystal structure of the phosphatase domain of Clostridium thermocellum Pnkp with Mn(2+) and citrate in the active site. The protein consists of a core binuclear metallo-phosphoesterase fold (exemplified by bacteriophage λ phosphatase) embellished by distinctive secondary structure elements. The active site contains a single Mn(2+) in an octahedral coordination complex with Asp187, His189, Asp233, two citrate oxygens and a water. The citrate fills the binding site for the scissile phosphate, wherein it is coordinated by Arg237, Asn263 and His264. The citrate invades the site normally occupied by a second metal (engaged by Asp233, Asn263, His323 and His376), and thereby dislocates His376. A continuous tract of positive surface potential flanking the active site suggests an RNA binding site. The structure illuminates a large body of mutational data regarding the metal and substrate specificity of Clostridium thermocellum Pnkp phosphatase.
Organizational Affiliation:
Molecular Biology Program, Sloan-Kettering Institute, New York, NY 10065, USA.