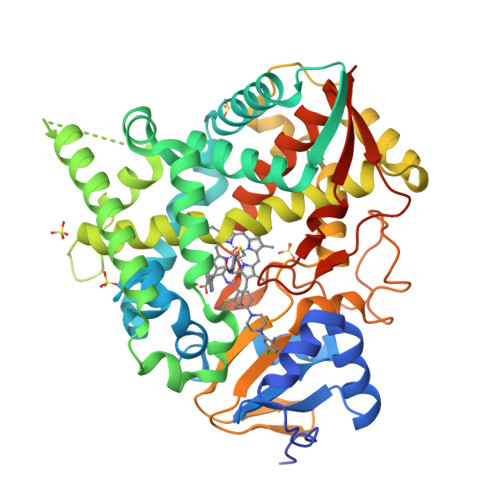
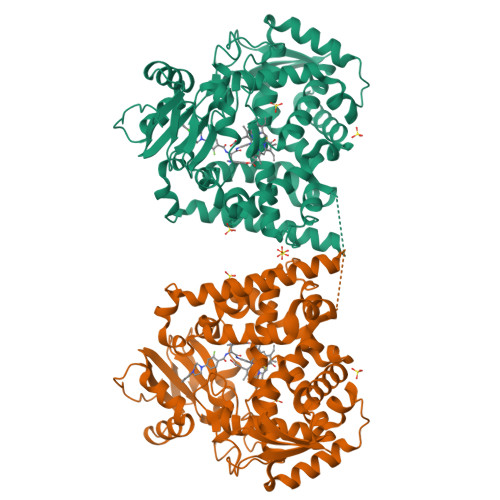
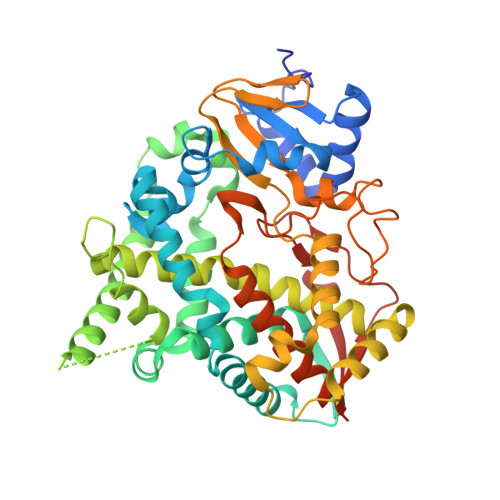
Binding Mode and Potency of N-Indolyl-Oxopyridinyl-4-Amino-Propanyl-Based Inhibitors Targeting Trypanosoma Cruzi Cyp51
Vieira, D.F., Choi, J.Y., Calvet, C.M., Siqueira-Neto, J.L., Johnston, J.B., Kellar, D., Gut, J., Cameron, M.D., Mckerrow, J.H., Roush, W.R., Podust, L.M.(2014) J Med Chem 57: 10162
- PubMed: 25393646
- DOI: https://doi.org/10.1021/jm501568b
- Primary Citation of Related Structures:
4C27, 4C28, 4UVR - PubMed Abstract:
Chagas disease is a chronic infection in humans caused by Trypanosoma cruzi and manifested in progressive cardiomyopathy and/or gastrointestinal dysfunction. Limited therapeutic options to prevent and treat Chagas disease put 8 million people infected with T. cruzi worldwide at risk. CYP51, involved in the biosynthesis of the membrane sterol component in eukaryotes, is a promising drug target in T. cruzi. We report the structure-activity relationships (SAR) of an N-arylpiperazine series of N-indolyloxopyridinyl-4-aminopropanyl-based inhibitors designed to probe the impact of substituents in the terminal N-phenyl ring on binding mode, selectivity and potency. Depending on the substituents at C-4, two distinct ring binding modes, buried and solvent-exposed, have been observed by X-ray structure analysis (resolution of 1.95-2.48 Å). The 5-chloro-substituted analogs 9 and 10 with no substituent at C-4 demonstrated improved selectivity and potency, suppressing ≥ 99.8% parasitemia in mice when administered orally at 25 mg/kg, b.i.d., for 4 days.
Organizational Affiliation:
Center for Discovery and Innovation in Parasitic Diseases, ‡Department of Pathology, and §Department of Pharmaceutical Chemistry, University of California-San Francisco , San Francisco, California 94158, United States.