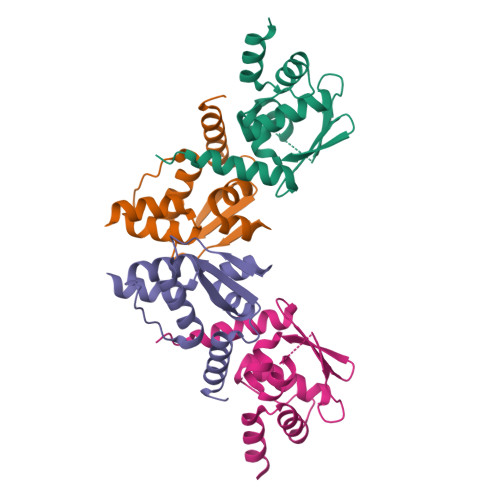
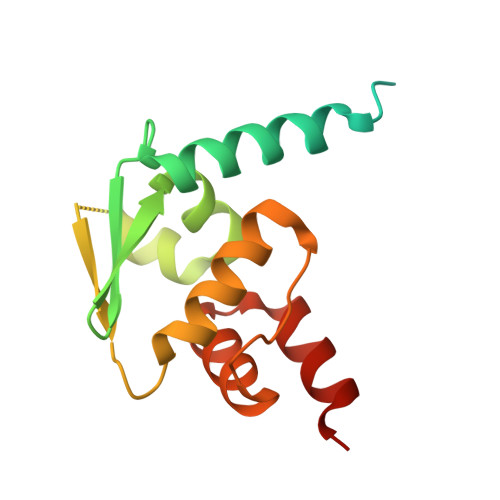
Polymerization of ZBTB transcription factors regulates chromatin occupancy.
Park, P.M.C., Park, J., Brown, J., Hunkeler, M., Roy Burman, S.S., Donovan, K.A., Yoon, H., Nowak, R.P., Slabicki, M., Ebert, B.L., Fischer, E.S.(2024) Mol Cell 84: 2511-2524.e8
- PubMed: 38996460
- DOI: https://doi.org/10.1016/j.molcel.2024.06.010
- Primary Citation of Related Structures:
9B9R, 9B9V - PubMed Abstract:
BCL6, an oncogenic transcription factor (TF), forms polymers in the presence of a small-molecule molecular glue that stabilizes a complementary interface between homodimers of BCL6's broad-complex, tramtrack, and bric-à-brac (BTB) domain. The BTB domains of other proteins, including a large class of TFs, have similar architectures and symmetries, raising the possibility that additional BTB proteins self-assemble into higher-order structures. Here, we surveyed 189 human BTB proteins with a cellular fluorescent reporter assay and identified 18 ZBTB TFs that show evidence of polymerization. Through biochemical and cryoelectron microscopy (cryo-EM) studies, we demonstrate that these ZBTB TFs polymerize into filaments. We found that BTB-domain-mediated polymerization of ZBTB TFs enhances chromatin occupancy within regions containing homotypic clusters of TF binding sites, leading to repression of target genes. Our results reveal a role of higher-order structures in regulating ZBTB TFs and suggest an underappreciated role for TF polymerization in modulating gene expression.
Organizational Affiliation:
Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA 02115, USA; Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA.