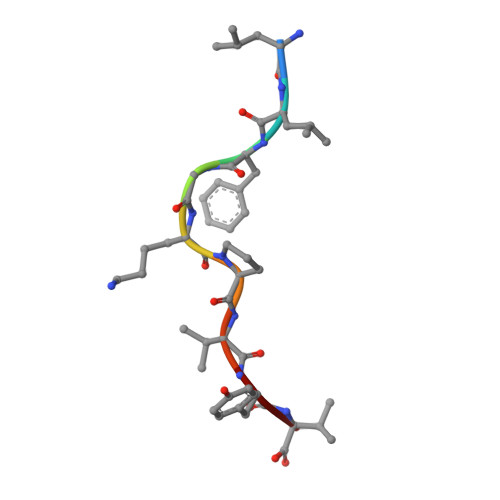
T Cell Receptor Recognition via Cooperative Conformational Plasticity.
Gagnon, S.J., Borbulevych, O.Y., Davis-Harrison, R.L., Turner, R.V., Damirjian, M., Wojnarowicz, A., Biddison, W.E., Baker, B.M.(2006) J Mol Biol 363: 228-243
- PubMed: 16962135
- DOI: https://doi.org/10.1016/j.jmb.2006.08.045
- Primary Citation of Related Structures:
2GIT, 2GJ6 - PubMed Abstract:
Although T cell receptor cross-reactivity is a fundamental property of the immune system and is implicated in numerous autoimmune pathologies, the molecular mechanisms by which T cell receptors can recognize and respond to diverse ligands are incompletely understood. In the current study we examined the response of the human T cell lymphotropic virus-1 (HTLV-1) Tax-specific T cell receptor (TCR) A6 to a panel of structurally distinct haptens coupled to the Tax 11-19 peptide with a lysine substitution at position 5 (Tax5K, LLFG[K-hapten]PVYV). The A6 TCR could cross-reactively recognize one of these haptenated peptides, Tax-5K-4-(3-Indolyl)-butyric acid (IBA), presented by HLA-A*0201. The crystal structures of Tax5K-IBA/HLA-A2 free and in complex with A6 reveal that binding is mediated by a mechanism of cooperative conformational plasticity involving conformational changes on both sides of the protein-protein interface, including the TCR complementarity determining region (CDR) loops, Valpha/Vbeta domain orientation, and the hapten-modified peptide. Our findings illustrate the complex role that protein dynamics can play in TCR cross-reactivity and highlight that T cell receptor recognition of ligand can be achieved through diverse and complex molecular mechanisms that can occur simultaneously in the interface, not limited to molecular mimicry and CDR loop shifts.
Organizational Affiliation:
Molecular Immunology Section, Neuroimmunology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA.