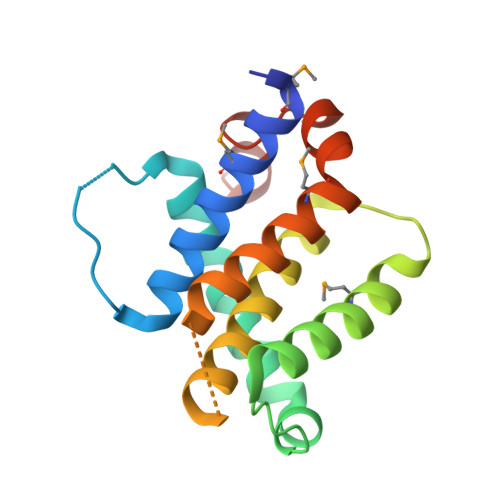
Structural Plasticity Underpins Promiscuous Binding of the Prosurvival Protein A1.
Smits, C., Czabotar, P.E., Hinds, M.G., Day, C.L.(2008) Structure 16: 818
- PubMed: 18462686
- DOI: https://doi.org/10.1016/j.str.2008.02.009
- Primary Citation of Related Structures:
2VOF, 2VOG, 2VOH, 2VOI - PubMed Abstract:
Apoptotic pathways are regulated by protein-protein interactions. Interaction of the BH3 domains of proapoptotic Bcl-2 family proteins with the hydrophobic groove of prosurvival proteins is critical. Whereas some BH3 domains bind in a promiscuous manner, others exhibit considerable selectivity and the sequence characteristics that distinguish these activities are unclear. In this study, crystal structures of complexes between the prosurvival protein A1 and the BH3 domains from Puma, Bmf, Bak, and Bid have been solved. The structure of A1 is similar to that of other prosurvival proteins, although features, such as an acidic patch in the binding groove, may allow specific therapeutic modulation of apoptosis. Significant conformational plasticity was observed in the intermolecular interactions and these differences explain some of the variation in affinity. This study, in combination with published data, suggests that interactions between conserved residues demarcate optimal binding.
Organizational Affiliation:
Biochemistry Department, University of Otago, Dunedin 9054, New Zealand.