Crystal structure of alpha-tubulin acetyltransferase domain of human Mec-17
Yuzawa, S., Sumimoto, H.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Alpha-tubulin N-acetyltransferase | 201 | Homo sapiens | Mutation(s): 0 Gene Names: ATAT1, C6orf134, MEC17, Nbla00487 EC: 2.3.1.108 | 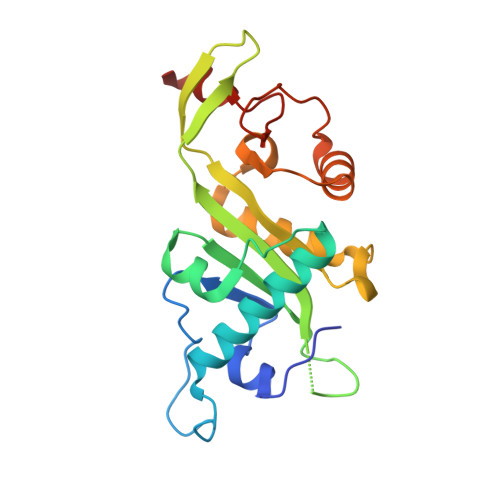 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q5SQI0 (Homo sapiens) Explore Q5SQI0 Go to UniProtKB: Q5SQI0 | |||||
PHAROS: Q5SQI0 GTEx: ENSG00000137343 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q5SQI0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ACO Query on ACO | B [auth A] | ACETYL COENZYME *A C23 H38 N7 O17 P3 S ZSLZBFCDCINBPY-ZSJPKINUSA-N |  | ||
| EDO Query on EDO | F [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| CL Query on CL | C [auth A], D [auth A], E [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 45.175 | α = 90 |
| b = 117.464 | β = 90 |
| c = 37.38 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |