Expanding the Binding Envelope of Cyp51 Inhibitors Targeting Trypanosoma Cruzi with 4-Aminopyridyl-Based Sulfonamide Derivatives
Vieira, D.F., Choi, J.Y., Roush, W.R., Podust, L.M.(2014) Chembiochem 15: 1111
- PubMed: 24771705
- DOI: https://doi.org/10.1002/cbic.201402027
- Primary Citation of Related Structures:
4COH - PubMed Abstract:
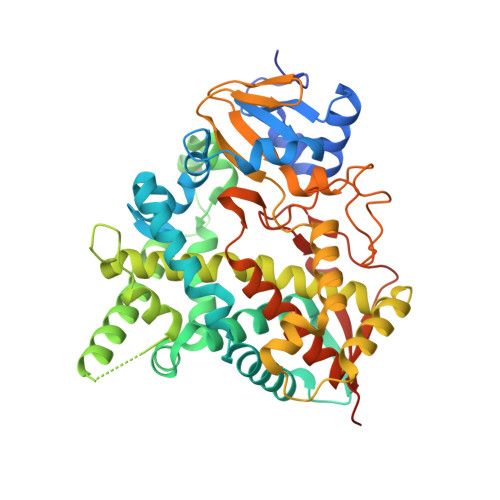
Chagas disease is a chronic infection caused by the protozoan parasite Trypanosoma cruzi, manifested in progressive cardiomyopathy and/or gastrointestinal dysfunction. Therapeutic options to prevent or treat Chagas disease are limited. CYP51, the enzyme key to the biosynthesis of eukaryotic membrane sterols, is a validated drug target in both fungi and T. cruzi. Sulfonamide derivatives of 4-aminopyridyl-based inhibitors of T. cruzi CYP51 (TcCYP51), including the sub-nanomolar compound 3, have molecular structures distinct from other validated CYP51 inhibitors. They augment the biologically relevant chemical space of molecules targeting TcCYP51. In a 2.08 Å X-ray structure, TcCYP51 is in a conformation that has been influenced by compound 3 and is distinct from the previously characterized ground-state conformation of CYP51 drug-target complexes. That the binding site was modulated in response to an incoming inhibitor for the first time characterizes TcCYP51 as a flexible target rather than a rigid template.
Organizational Affiliation:
Department of Pathology, Center for Discovery and Innovation in Parasitic Diseases, University of California San Francisco, 1700 4th Street, San Francisco, CA 94158 (USA).