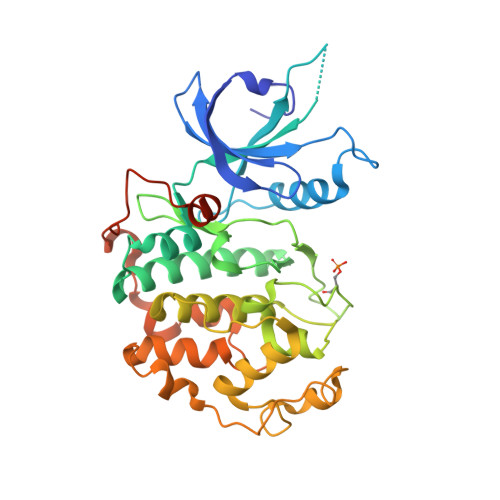
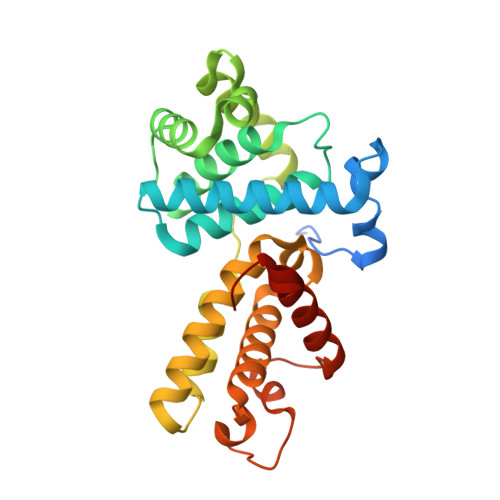
The structure and substrate specificity of human Cdk12/Cyclin K.
Bosken, C.A., Farnung, L., Hintermair, C., Merzel Schachter, M., Vogel-Bachmayr, K., Blazek, D., Anand, K., Fisher, R.P., Eick, D., Geyer, M.(2014) Nat Commun 5: 3505-3505
- PubMed: 24662513
- DOI: https://doi.org/10.1038/ncomms4505
- Primary Citation of Related Structures:
4NST - PubMed Abstract:
Phosphorylation of the RNA polymerase II C-terminal domain (CTD) by cyclin-dependent kinases is important for productive transcription. Here we determine the crystal structure of Cdk12/CycK and analyse its requirements for substrate recognition. Active Cdk12/CycK is arranged in an open conformation similar to that of Cdk9/CycT but different from those of cell cycle kinases. Cdk12 contains a C-terminal extension that folds onto the N- and C-terminal lobes thereby contacting the ATP ribose. The interaction is mediated by an HE motif followed by a polybasic cluster that is conserved in transcriptional CDKs. Cdk12/CycK showed the highest activity on a CTD substrate prephosphorylated at position Ser7, whereas the common Lys7 substitution was not recognized. Flavopiridol is most potent towards Cdk12 but was still 10-fold more potent towards Cdk9. T-loop phosphorylation of Cdk12 required coexpression with a Cdk-activating kinase. These results suggest the regulation of Pol II elongation by a relay of transcriptionally active CTD kinases.
- 1] Group Physical Biochemistry, Center of Advanced European Studies and Research, Ludwig-Erhard-Allee 2, Bonn 53175, Germany [2] Department of Physical Biochemistry, Max Planck Institute of Molecular Physiology, Otto-Hahn-Strasse 11, Dortmund 44227, Germany.
Organizational Affiliation: