Formation of Amyloid Fibers by Monomeric Light-Chain Variable Domains.
Brumshtein, B., Esswein, S.R., Landau, M., Ryan, C.M., Whitelegge, J.P., Phillips, M.L., Cascio, D., Sawaya, M.R., Eisenberg, D.S.(2014) J Biol Chem 289: 27513
- PubMed: 25138218
- DOI: https://doi.org/10.1074/jbc.M114.585638
- Primary Citation of Related Structures:
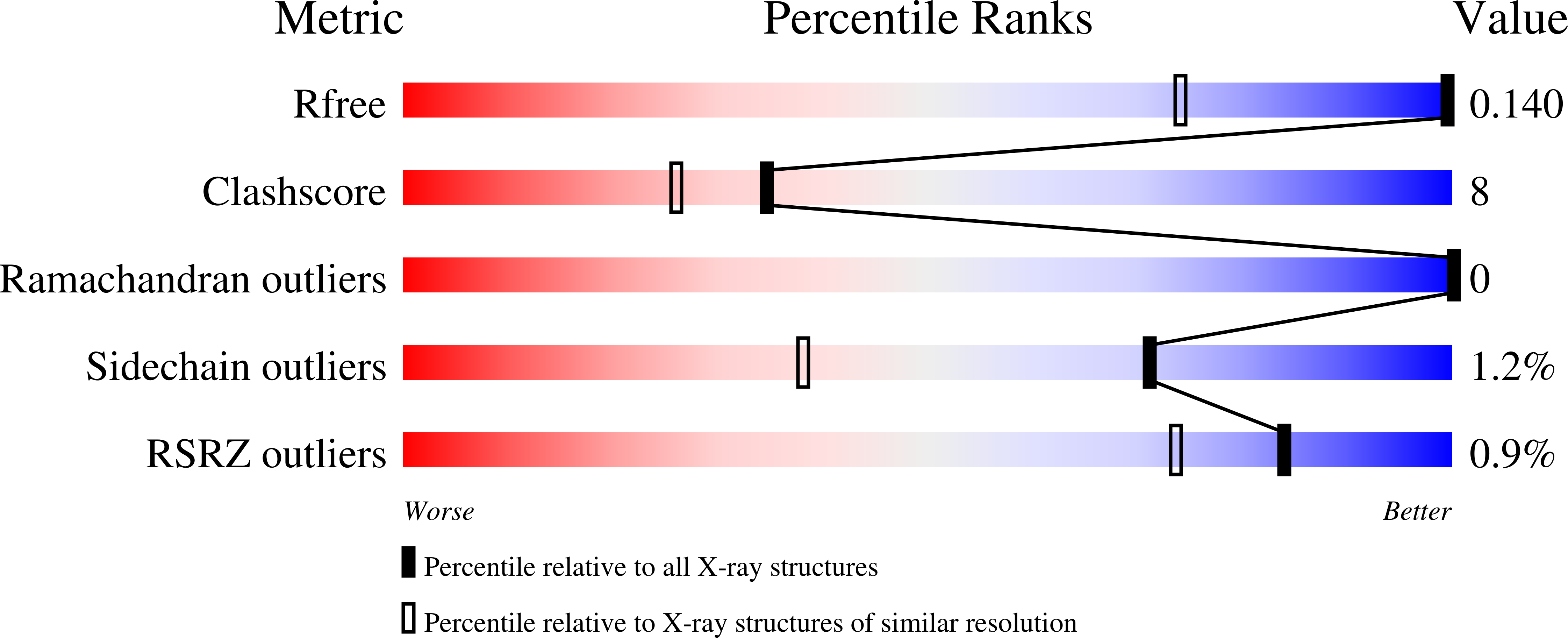
4UNT, 4UNU, 4UNV - PubMed Abstract:
Systemic light chain amyloidosis is a lethal disease characterized by excess immunoglobulin light chains and light chain fragments composed of variable domains, which aggregate into amyloid fibers. These fibers accumulate and damage organs. Some light chains induce formation of amyloid fibers, whereas others do not, making it unclear what distinguishes amyloid formers from non-formers. One mechanism by which sequence variation may reduce propensity to form amyloid fibers is by shifting the equilibrium toward an amyloid-resistant quaternary structure. Here we identify the monomeric form of the Mcg immunoglobulin light chain variable domain as the quaternary unit required for amyloid fiber assembly. Dimers of Mcg variable domains remain stable and soluble, yet become prone to assemble into amyloid fibers upon disassociation into monomers.
Organizational Affiliation:
From the Departments of Biological Chemistry and Chemistry and Biochemistry, Howard Hughes Medical Institute, UCLA-Department of Energy (DOE) Institute for Genomics and Proteomics, UCLA, Los Angeles, California 90095 and.