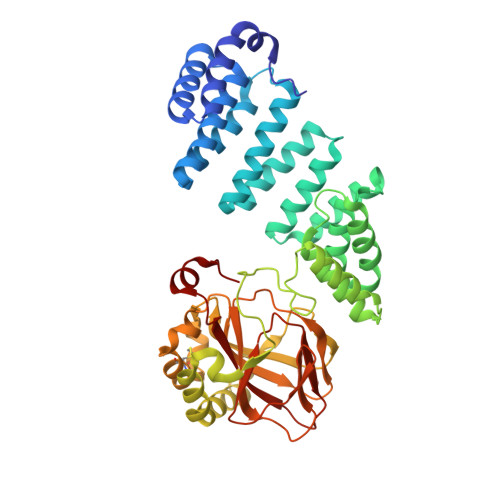
Aspartate/asparagine-beta-hydroxylase: a high-throughput mass spectrometric assay for discovery of small molecule inhibitors.
Brewitz, L., Tumber, A., Pfeffer, I., McDonough, M.A., Schofield, C.J.(2020) Sci Rep 10: 8650-8650
- PubMed: 32457455
- DOI: https://doi.org/10.1038/s41598-020-65123-9
- Primary Citation of Related Structures:
5JTC - PubMed Abstract:
The human 2-oxoglutarate dependent oxygenase aspartate/asparagine-β-hydroxylase (AspH) catalyses the hydroxylation of Asp/Asn-residues in epidermal growth factor-like domains (EGFDs). AspH is upregulated on the surface of malign cancer cells; increased AspH levels correlate with tumour invasiveness. Due to a lack of efficient assays to monitor the activity of isolated AspH, there are few reports of studies aimed at identifying small-molecule AspH inhibitors. Recently, it was reported that AspH substrates have a non-canonical EGFD disulfide pattern. Here we report that a stable synthetic thioether mimic of AspH substrates can be employed in solid phase extraction mass spectrometry based high-throughput AspH inhibition assays which are of excellent robustness, as indicated by high Z'-factors and good signal-to-noise/background ratios. The AspH inhibition assay was applied to screen approximately 1500 bioactive small-molecules, including natural products and active pharmaceutical ingredients of approved human therapeutics. Potent AspH inhibitors were identified from both compound classes. Our AspH inhibition assay should enable the development of potent and selective small-molecule AspH inhibitors and contribute towards the development of safer inhibitors for other 2OG oxygenases, e.g. screens of the hypoxia-inducible factor prolyl-hydroxylase inhibitors revealed that vadadustat inhibits AspH with moderate potency.
- Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, OX1 3TA, Oxford, United Kingdom.
Organizational Affiliation: