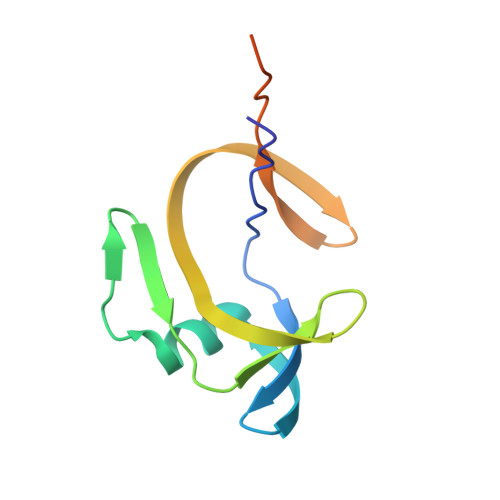
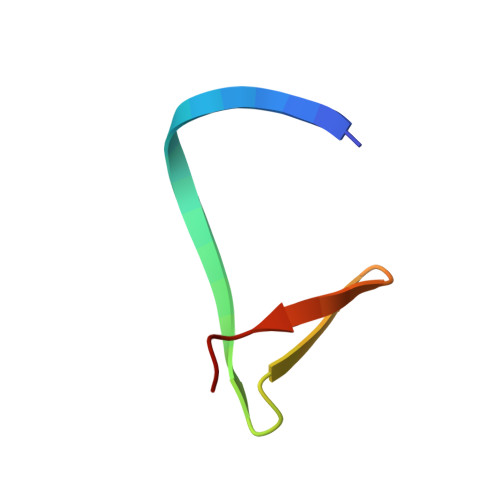
Structural Insights into Subunits Assembly and the Oxyester Splicing Mechanism of Neq pol Split Intein.
Gordo, V., Aparicio, D., Perez-Luque, R., Benito, A., Vilanova, M., Uson, I., Fita, I., Ribo, M.(2018) Cell Chem Biol 25: 871-879.e2
- PubMed: 29754955
- DOI: https://doi.org/10.1016/j.chembiol.2018.04.008
- Primary Citation of Related Structures:
5OXW, 5OXX, 5OXZ - PubMed Abstract:
Split inteins are expressed as two separated subunits (N-intein and C-intein) fused to the corresponding exteins. The specific association of both intein subunits precedes protein splicing, which results in excision of the intein subunits and in ligation, by a peptide bond, of the concomitant exteins. Catalytically active intein precursors are typically too reactive for crystallization or even isolation. Neq pol is the trans-intein of the B-type DNA polymerase I split gene from hyperthermophile Nanoarchaeum equitans. We have determined the crystal structures of both the isolated NeqN and the complex of NeqN and NeqC subunits carrying the wild-type sequences, including the essential catalytic residues Ser1 and Thr+1, in addition to seven and three residues of the N- and C-exteins, respectively. These structures provide detailed information on the unique oxyester chemistry of the splicing mechanism of Neq pol and of the extensive rearrangements that occur in NeqN during the association step.
- Laboratori d'Enginyeria de Proteïnes, Departament de Biologia, Facultat de Ciències, Universitat de Girona, C/ Maria Aurèlia Capmany 40, 17003 Girona, Spain; IdIBGi Hospital Universitari Josep Trueta, Girona, Spain.
Organizational Affiliation: