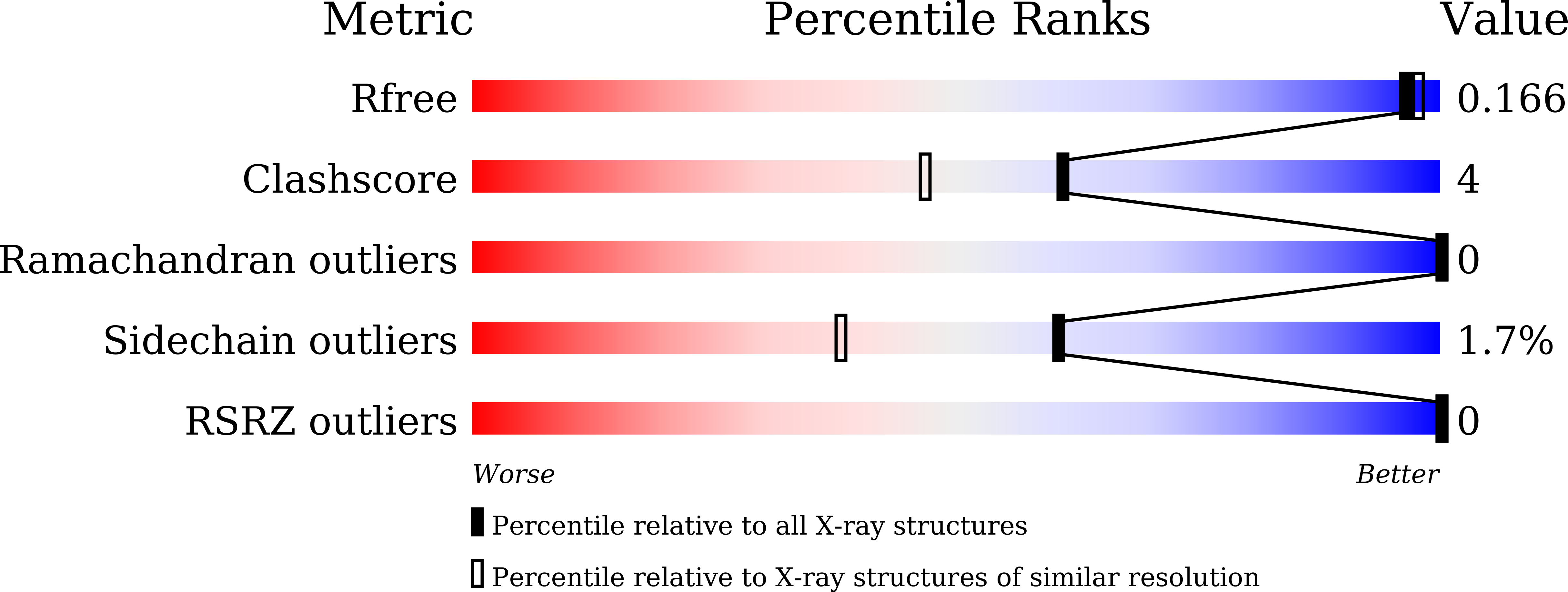
Crystal Structure of Catechol O-Methyltransferase Complexed with Nitecapone.
Iijima, H., Takebe, K., Suzuki, M., Kobayashi, H., Takamiya, T., Saito, H., Niwa, N., Kuwada-Kusunose, T.(2020) Chem Pharm Bull (Tokyo) 68: 447-451
- PubMed: 32378542
- DOI: https://doi.org/10.1248/cpb.c20-00011
- Primary Citation of Related Structures:
6LFE - PubMed Abstract:
Catechol O-methyltransferase (COMT) is known as an important drug-target protein in the field of Parkinson's disease. All clinically approved COMT inhibitors bring a 5-substituted-3-nitrocatechol ring as a pharmacophore, and they bind to COMT with S-adenosylmethionine (SAM) and an Mg 2+ ion to form a quaternary complex (COMT/SAM/Mg 2+ /inhibitor). However, structural information about such quaternary complexes is only available for a few inhibitors. Here, a new crystal structure of COMT complexed with nitecapone (5), SAM and Mg 2+ is revealed. Comparison of the structures of these complexes indicates that conformation of the catechol binding pocket is almost constant regardless of structure of the inhibitors. The only restriction of the side chain of inhibitors (i.e., the substituent at the 5-position of 3-nitrocatechol) seems to be that it does not make steric repulsion with COMT. However, recent crystallographic and biochemical studies suggest that COMT is a flexible protein, and its conformational flexibility seems crucial for its catalytic process. Based on this information, implications of these quaternary inhibitor complexes were investigated. Met 40 in the α2α3-loop makes atomic contacts with SAM or S-adenosylhomocysteine and the 3-position of the catechol inhibitor. This interaction seems to play a critical role in the affinity of the inhibitor and to stabilize the COMT/SAM/Mg 2+ /nitrocatechol inhibitor complex by fixing the flexible α2α3-loop.
Organizational Affiliation:
School of Pharmacy, Nihon University.