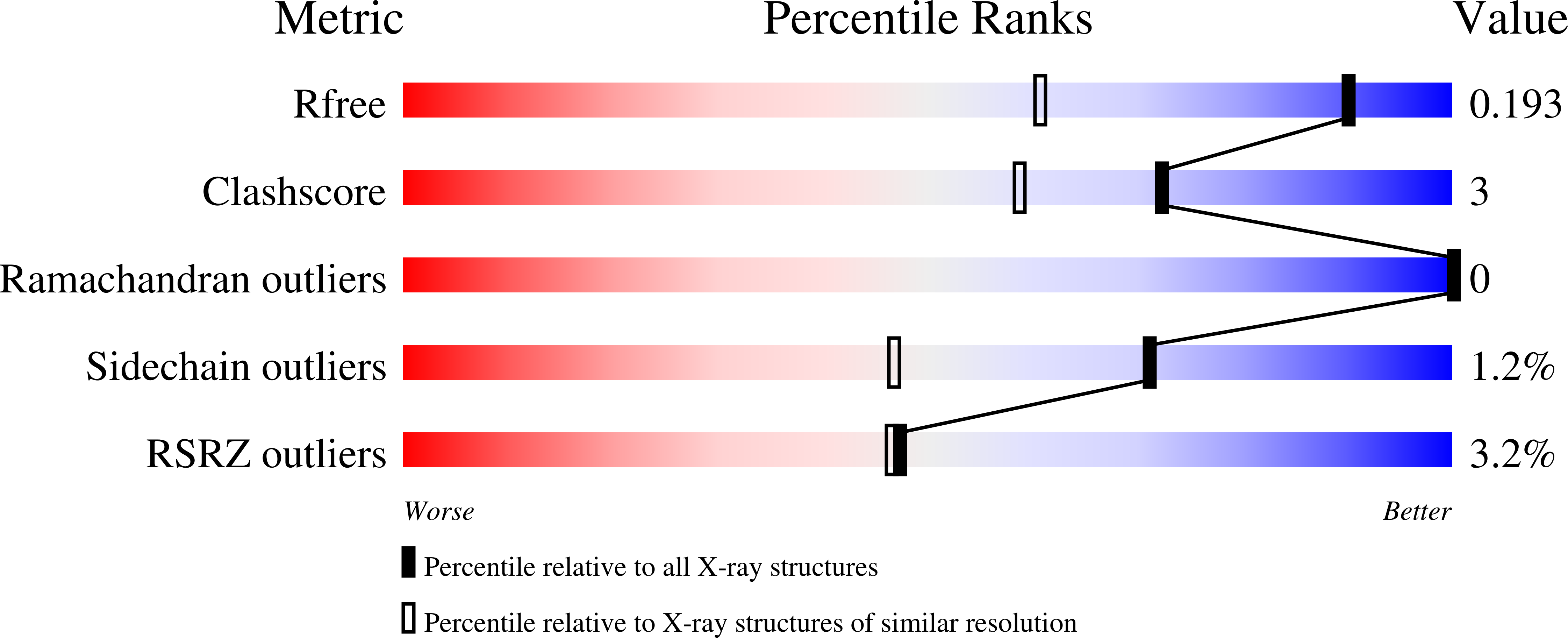
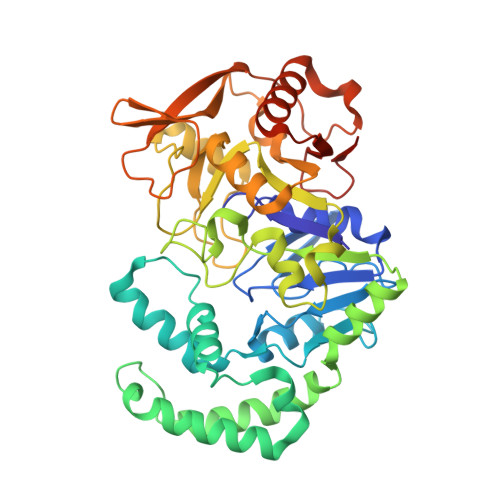
The pursuit of new alternative ways to eradicate Helicobacter pylori continues: Detailed characterization of interactions in the adenylosuccinate synthetase active site
Bubic, A., Narczyk, M., Petek, A., Wojtys, M.I., Maksymiuk, W., Wielgus-Kutrowska, B., Winiewska-Szajewska, M., Pavkov-Keller, T., Bertosa, B., Stefanic, Z., Luic, M., Bzowska, A., Lescic Asler, I.(2023) Int J Biol Macromol