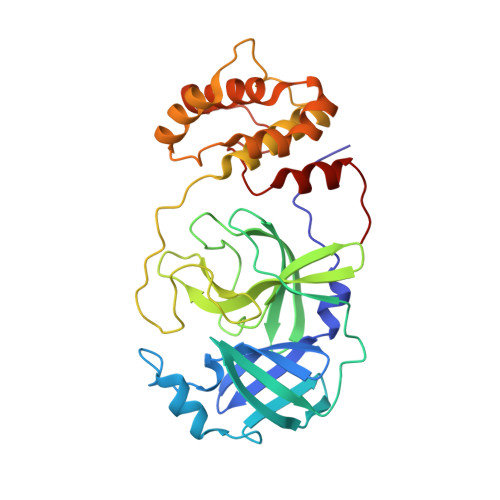
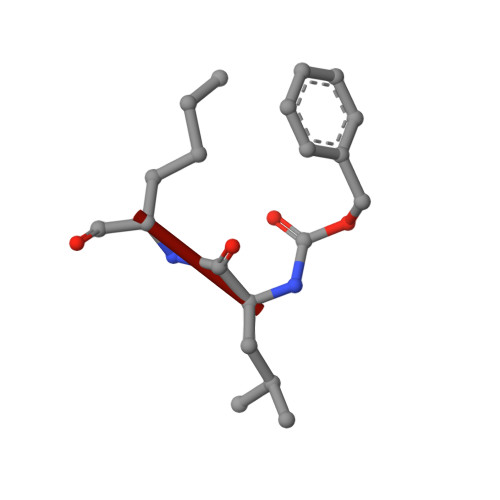
X-ray screening identifies active site and allosteric inhibitors of SARS-CoV-2 main protease.
Gunther, S., Reinke, P.Y.A., Fernandez-Garcia, Y., Lieske, J., Lane, T.J., Ginn, H.M., Koua, F.H.M., Ehrt, C., Ewert, W., Oberthuer, D., Yefanov, O., Meier, S., Lorenzen, K., Krichel, B., Kopicki, J.D., Gelisio, L., Brehm, W., Dunkel, I., Seychell, B., Gieseler, H., Norton-Baker, B., Escudero-Perez, B., Domaracky, M., Saouane, S., Tolstikova, A., White, T.A., Hanle, A., Groessler, M., Fleckenstein, H., Trost, F., Galchenkova, M., Gevorkov, Y., Li, C., Awel, S., Peck, A., Barthelmess, M., Schlunzen, F., Lourdu Xavier, P., Werner, N., Andaleeb, H., Ullah, N., Falke, S., Srinivasan, V., Franca, B.A., Schwinzer, M., Brognaro, H., Rogers, C., Melo, D., Zaitseva-Doyle, J.J., Knoska, J., Pena-Murillo, G.E., Mashhour, A.R., Hennicke, V., Fischer, P., Hakanpaa, J., Meyer, J., Gribbon, P., Ellinger, B., Kuzikov, M., Wolf, M., Beccari, A.R., Bourenkov, G., von Stetten, D., Pompidor, G., Bento, I., Panneerselvam, S., Karpics, I., Schneider, T.R., Garcia-Alai, M.M., Niebling, S., Gunther, C., Schmidt, C., Schubert, R., Han, H., Boger, J., Monteiro, D.C.F., Zhang, L., Sun, X., Pletzer-Zelgert, J., Wollenhaupt, J., Feiler, C.G., Weiss, M.S., Schulz, E.C., Mehrabi, P., Karnicar, K., Usenik, A., Loboda, J., Tidow, H., Chari, A., Hilgenfeld, R., Uetrecht, C., Cox, R., Zaliani, A., Beck, T., Rarey, M., Turk, D., Hinrichs, W., Chapman, H.N., Pearson, A.R., Betzel, C., Meents, A.(2021) Science 372: 642-646
- PubMed: 33811162
- DOI: https://doi.org/10.1126/science.abf7945
- Primary Citation of Related Structures:
6YNQ, 6YVF, 7A1U, 7ABU, 7ADW, 7AF0, 7AGA, 7AHA, 7AK4, 7AKU, 7AMJ, 7ANS, 7AOL, 7AP6, 7APH, 7AQE, 7AQI, 7AQJ, 7AR5, 7AR6, 7ARF, 7AVD, 7AWR, 7AWS, 7AWU, 7AWW, 7AX6, 7AXM, 7AXO, 7AY7, 7B83, 7NEV - PubMed Abstract:
The coronavirus disease (COVID-19) caused by SARS-CoV-2 is creating tremendous human suffering. To date, no effective drug is available to directly treat the disease. In a search for a drug against COVID-19, we have performed a high-throughput x-ray crystallographic screen of two repurposing drug libraries against the SARS-CoV-2 main protease (M pro ), which is essential for viral replication. In contrast to commonly applied x-ray fragment screening experiments with molecules of low complexity, our screen tested already-approved drugs and drugs in clinical trials. From the three-dimensional protein structures, we identified 37 compounds that bind to M pro In subsequent cell-based viral reduction assays, one peptidomimetic and six nonpeptidic compounds showed antiviral activity at nontoxic concentrations. We identified two allosteric binding sites representing attractive targets for drug development against SARS-CoV-2.
- Center for Free-Electron Laser Science, Deutsches Elektronen-Synchrotron DESY, Notkestr. 85, 22607 Hamburg, Germany. sebastian.guenther@desy.de alke.meents@desy.de.
Organizational Affiliation: