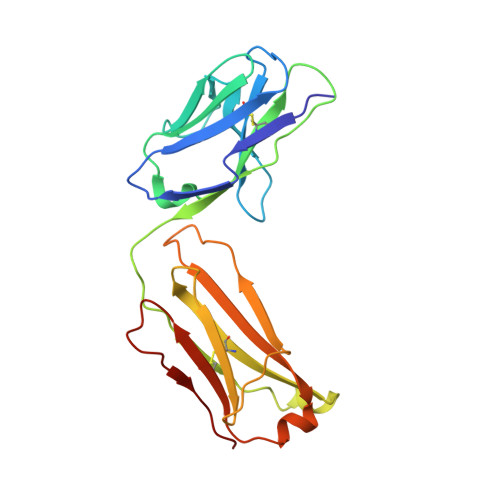
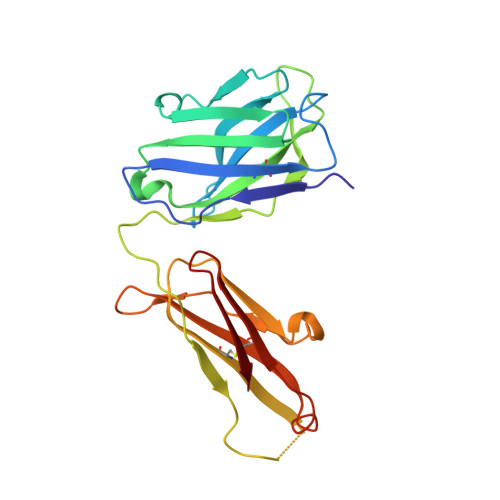
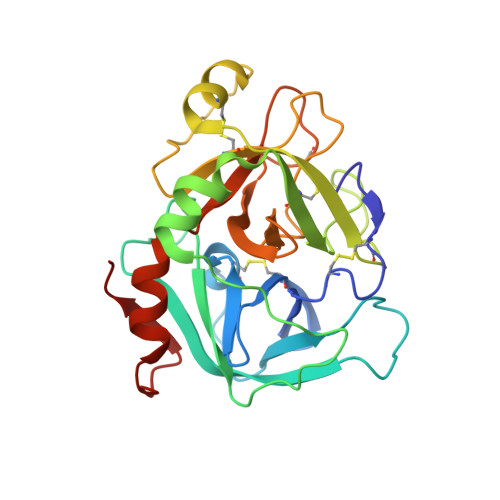
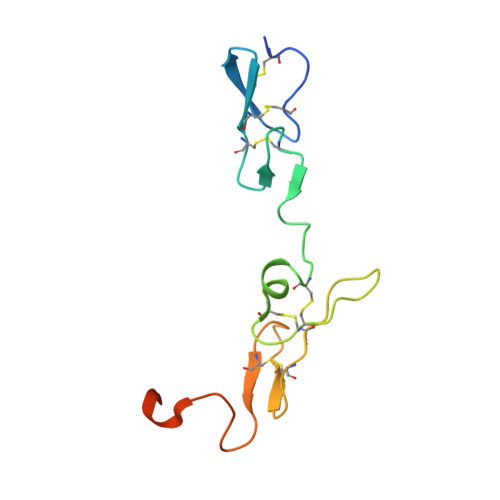
A bispecific antibody approach for the potential prophylactic treatment of inherited bleeding disorders.
Gandhi, P.S., Zivkovic, M., Ostergaard, H., Bonde, A.C., Elm, T., Lovgreen, M.N., Schluckebier, G., Johansson, E., Olsen, O.H., Olsen, E.H.N., de Bus, I.A., Bloem, K., Alskar, O., Rea, C.J., Bjorn, S.E., Schutgens, R.E., Sorensen, B., Urbanus, R.T., Faber, J.H.(2024) Nat Cardiovasc Res 3: 166-185
- PubMed: 39196196
- DOI: https://doi.org/10.1038/s44161-023-00418-4
- Primary Citation of Related Structures:
8CHE, 8CN9 - PubMed Abstract:
Inherited bleeding disorders such as Glanzmann thrombasthenia (GT) lack prophylactic treatment options. As a result, serious bleeding episodes are treated acutely with blood product transfusions or frequent, repeated intravenous administration of recombinant activated coagulation factor VII (rFVIIa). Here we describe HMB-001, a bispecific antibody designed to bind and accumulate endogenous FVIIa and deliver it to sites of vascular injury by targeting it to the TREM (triggering receptor expressed on myeloid cells)-like transcript-1 (TLT-1) receptor that is selectively expressed on activated platelets. In healthy nonhuman primates, HMB-001 prolonged the half-life of endogenous FVIIa, resulting in its accumulation. Mouse bleeding studies confirmed antibody-mediated potentiation of FVIIa hemostatic activity by TLT-1 targeting. In ex vivo models of GT, HMB-001 localized FVIIa on activated platelets and potentiated fibrin-dependent platelet aggregation. Taken together, these results indicate that HMB-001 has the potential to offer subcutaneous prophylactic treatment to prevent bleeds in people with GT and other inherited bleeding disorders, with a low-frequency dosing regimen.
Organizational Affiliation:
Hemab Therapeutics, Copenhagen, Denmark. prafull@hemab.com.