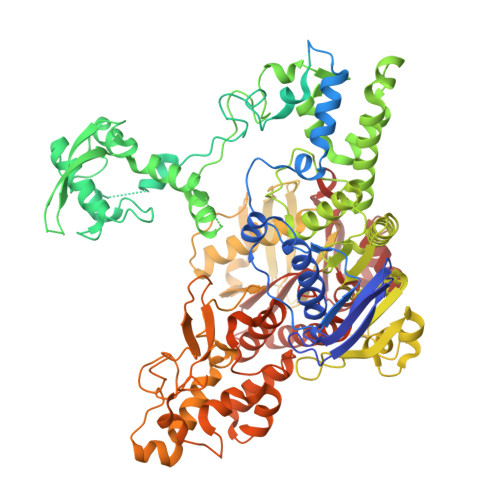
Structural snapshots of the mechanism of ATP-dependent DNA damage recognition by UvrA.
Nirwal, S., Czarnocki-Cieciura, M., Zajko, W., Skowronek, K., Szczepanowski, R.H., Nowotny, M.(2025) Nat Commun
- PubMed: 41381534
- DOI: https://doi.org/10.1038/s41467-025-67075-y
- Primary Citation of Related Structures:
9GXK, 9GXL, 9GXM, 9GXN, 9GXO, 9GXP, 9GXQ, 9GXR, 9GXS, 9GXT, 9GXU, 9GXV, 9GXW, 9GY7 - PubMed Abstract:
Nucleotide excision repair is a DNA repair pathway which detects and fixes various DNA lesions that distort the structure of DNA. In bacteria, the pathway starts with the UvrA protein which has two adenosine triphosphatase modules and forms dimers. The DNA is handed over from UvrA to UvrB, which is a weak helicase that verifies the presence of damage. Despite intense studies, the role of the ATPase activity of UvrA in damage recognition is unclear. Here, we present a series of cryo-electron microscopy structures of UvrA in complex with three different DNAs and in the presence and absence of nucleotides. We also present a structure of UvrA:UvrB:DNA complex. These structures reveal a major rearrangement of the UvrA dimer upon ATP binding. We propose that these conformational changes are used to mechanically probe the integrity of DNA for damage localization. Collectively, our results present snapshots of UvrA's ATP-dependent DNA damage detection.
- Laboratory of Protein Structure, International Institute of Molecular and Cell Biology, Warsaw, Poland.
Organizational Affiliation: