Neuroglobins: Pivotal Proteins Associated with Emerging Neural Systems and Precursors of Metazoan Globin Diversity.
Lechauve, C., Jager, M., Laguerre, L., Kiger, L., Correc, G., Leroux, C., Vinogradov, S., Czjzek, M., Marden, M.C., Bailly, X.(2013) J Biological Chem 288: 6957
- PubMed: 23288852
- DOI: https://doi.org/10.1074/jbc.M112.407601
- Primary Citation of Related Structures:
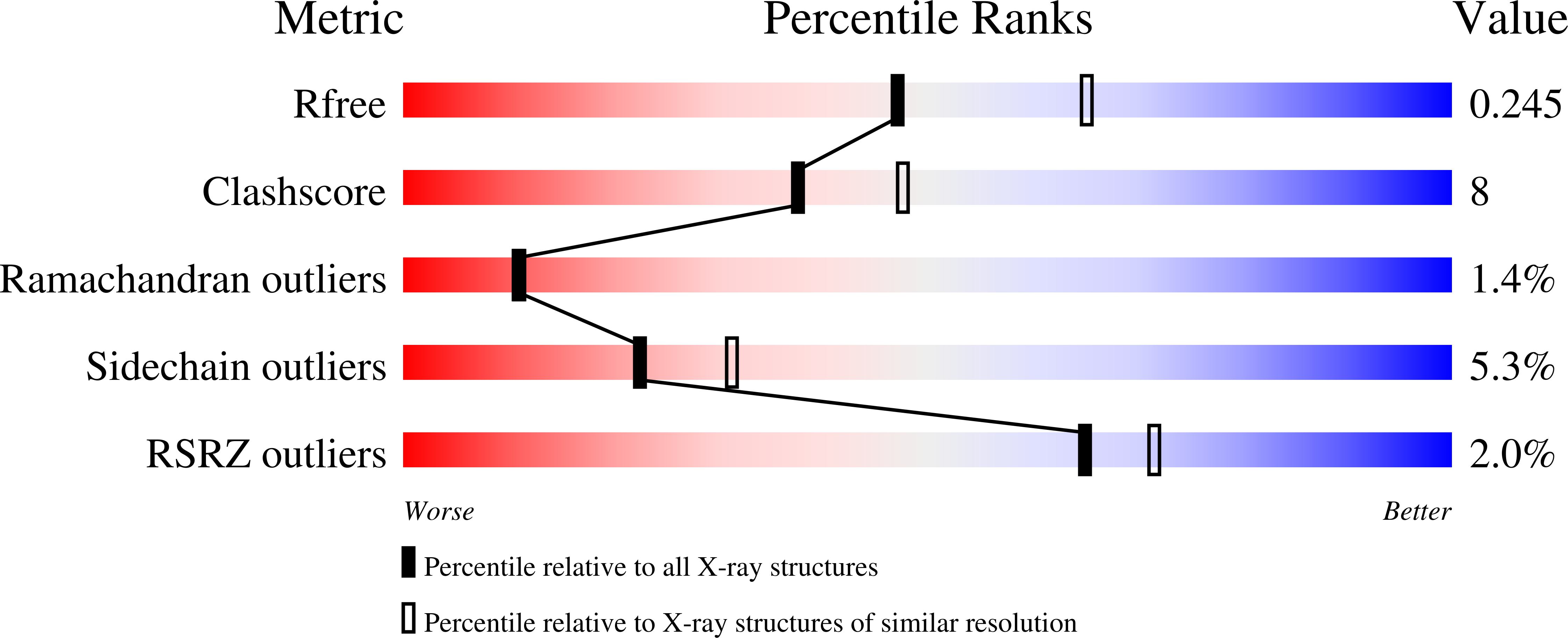
4B4Y - PubMed Abstract:
Neuroglobins, previously thought to be restricted to vertebrate neurons, were detected in the brain of a photosymbiotic acoel, Symsagittifera roscoffensis, and in neurosensory cells of the jellyfish Clytia hemisphaerica. For the neuroglobin of S. roscoffensis, a member of a lineage that originated either at the base of the bilateria or of the deuterostome clade, we report the ligand binding properties, crystal structure at 2.3 Å, and brain immunocytochemical pattern. We also describe in situ hybridizations of two neuroglobins specifically expressed in differentiating nematocytes (neurosensory cells) and in statocytes (ciliated mechanosensory cells) of C. hemisphaerica, a member of the early branching animal phylum cnidaria. In silico searches using these neuroglobins as queries revealed the presence of previously unidentified neuroglobin-like sequences in most metazoan lineages. Because neural systems are almost ubiquitous in metazoa, the constitutive expression of neuroglobin-like proteins strongly supports the notion of an intimate association of neuroglobins with the evolution of animal neural systems and hints at the preservation of a vitally important function. Neuroglobins were probably recruited in the first protoneurons in early metazoans from globin precursors. Neuroglobins were identified in choanoflagellates, sponges, and placozoans and were conserved during nervous system evolution. Because the origin of neuroglobins predates the other metazoan globins, it is likely that neuroglobin gene duplication followed by co-option and subfunctionalization led to the emergence of globin families in protostomes and deuterostomes (i.e. convergent evolution).
Organizational Affiliation:
INSERM, UMR S 968, Centre National de la Recherche Scientifique (CNRS), Unité Mixte de Recherche (UMR)_7210, Institut de la Vision Université Pierre et Marie Curie (UPMC)/Centre Hospitalier National d'Ophtalmologie des Quinze-Vingts, 17 rue Moreau, 75012 Paris, France.