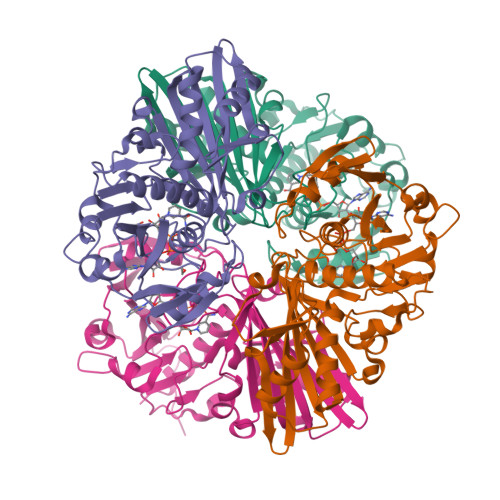
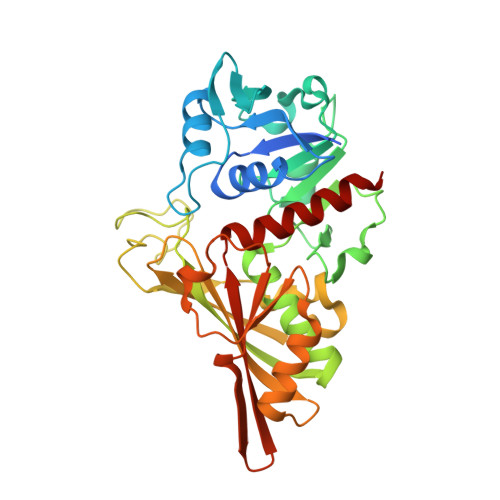
Structure of Streptococcus agalactiae glyceraldehyde-3-phosphate dehydrogenase holoenzyme reveals a novel surface.
Ayres, C.A., Schormann, N., Senkovich, O., Fry, A., Banerjee, S., Ulett, G.C., Chattopadhyay, D.(2014) Acta Crystallogr F Struct Biol Commun 70: 1333-1339
- PubMed: 25286935
- DOI: https://doi.org/10.1107/S2053230X14019517
- Primary Citation of Related Structures:
4QX6 - PubMed Abstract:
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a conserved cytosolic enzyme, which plays a key role in glycolysis. GAPDH catalyzes the oxidative phosphorylation of D-glyceraldehyde 3-phosphate using NAD or NADP as a cofactor. In addition, GAPDH localized on the surface of some bacteria is thought to be involved in macromolecular interactions and bacterial pathogenesis. GAPDH on the surface of group B streptococcus (GBS) enhances bacterial virulence and is a potential vaccine candidate. Here, the crystal structure of GBS GAPDH from Streptococcus agalactiae in complex with NAD is reported at 2.46 Å resolution. Although the overall structure of GBS GAPDH is very similar to those of other GAPDHs, the crystal structure reveals a significant difference in the area spanning residues 294-307, which appears to be more acidic. The amino-acid sequence of this region of GBS GAPDH is also distinct compared with other GAPDHs. This region therefore may be of interest as an immunogen for vaccine development.
Organizational Affiliation:
Science and Technology Honors Program, University of Alabama at Birmingham, Birmingham, AL 35294, USA.