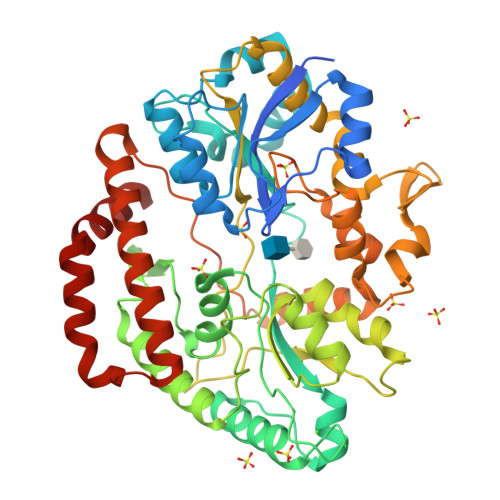
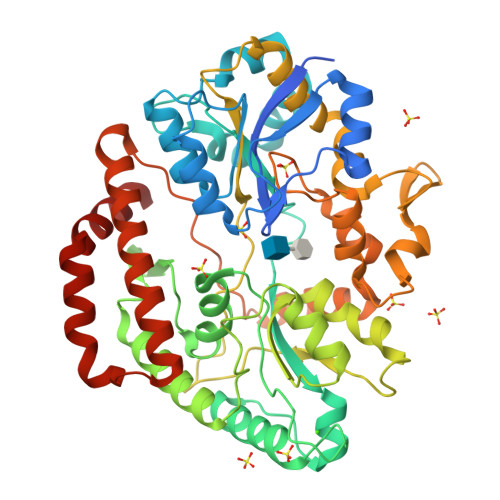
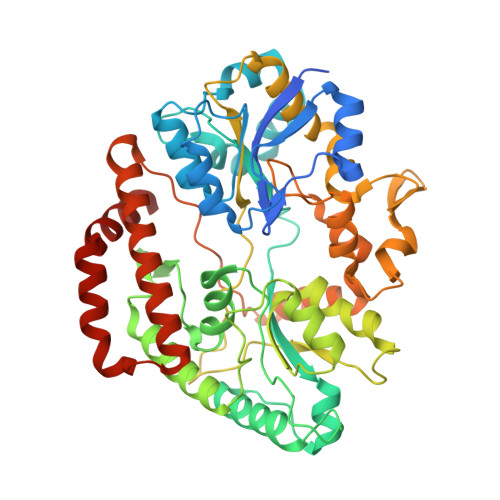
Substrate recognition by bacterial solute-binding protein is responsible for import of extracellular hyaluronan and chondroitin sulfate from the animal host.
Oiki, S., Sato, M., Mikami, B., Murata, K., Hashimoto, W.(2019) Biosci Biotechnol Biochem 83: 1946-1954
- PubMed: 31204616
- DOI: https://doi.org/10.1080/09168451.2019.1630250
- Primary Citation of Related Structures:
6INZ - PubMed Abstract:
Glycosaminoglycans (GAGs) such as hyaluronan and chondroitin in animal extracellular matrices contain disaccharide-repeating units. In a Gram-negative pathogenic Streptobacillus moniliformis , which belongs to Fusobacteria phylum and resides in rodent oral cavities, the solute-binding protein (Smon0123)-dependent ATP-binding cassette transporter imports unsaturated hyaluronan/chondroitin disaccharides into the cytoplasm after GAG lyase-dependent depolymerization. Here we show substrate recognition of unsaturated hyaluronan disaccharide by Smon0123. Moreover, Smon0123 exhibited no affinity for unsaturated chondroitin disaccharides containing three sulfate groups, distinct from non-sulfated, mono-sulfated, and di-sulfated chondroitin disaccharides previously identified as substrates. Crystal structure of Smon0123 with unsaturated hyaluronan disaccharide demonstrates that several residues, including Trp284 and Glu410, are crucial for binding to unsaturated hyaluronan/chondroitin disaccharides, whereas arrangements of water molecules at binding sites are found to be substrate dependent through comparison with substrate-bound structures determined previously. These residues are well conserved in Smon0123-like proteins of fusobacteria, and probably facilitate the fusobacterial residence in hyaluronan-rich oral cavities.
Organizational Affiliation:
Laboratory of Basic and Applied Molecular Biotechnology, Division of Food Science and Biotechnology, Graduate School of Agriculture, Kyoto University , Kyoto , Japan.