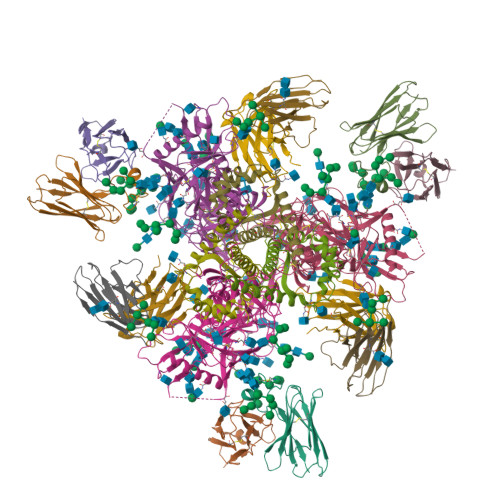
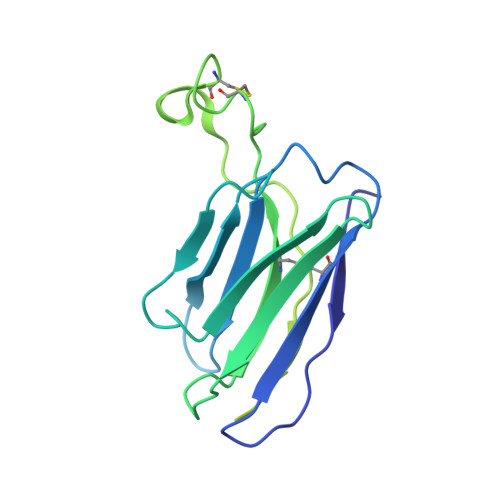
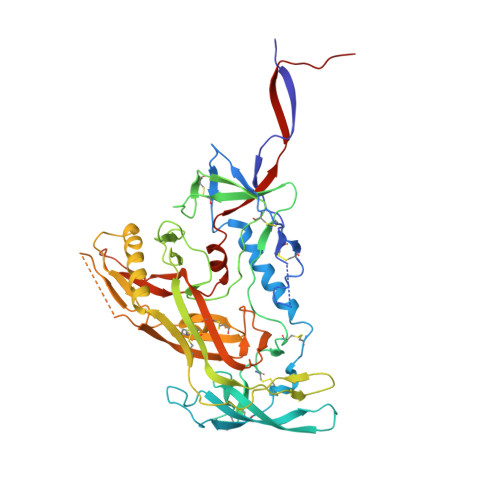
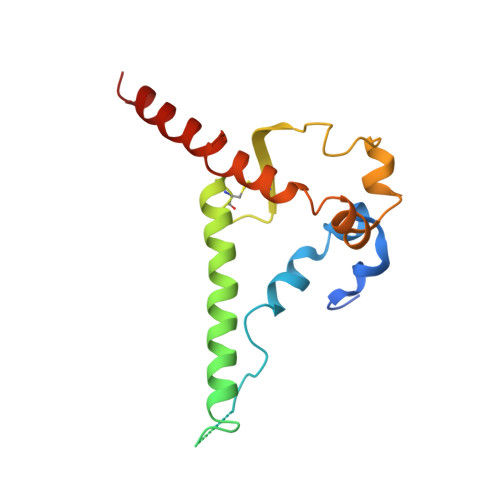
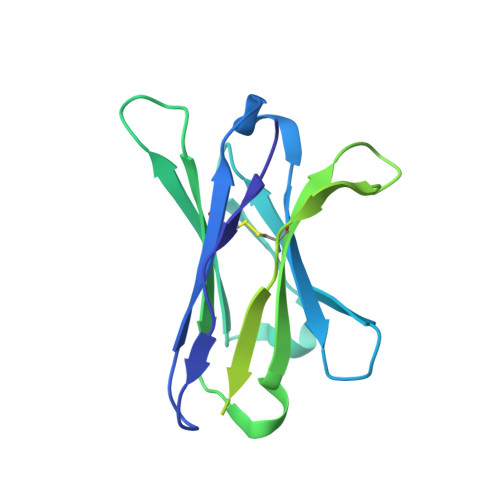
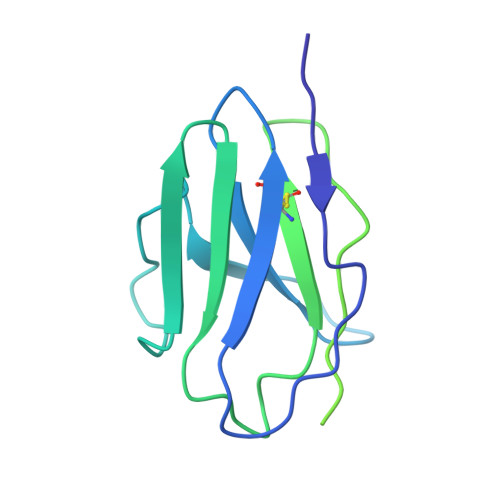
Epitope convergence of broadly HIV-1 neutralizing IgA and IgG antibody lineages in a viremic controller.
Lorin, V., Fernandez, I., Masse-Ranson, G., Bouvin-Pley, M., Molinos-Albert, L.M., Planchais, C., Hieu, T., Pehau-Arnaudet, G., Hrebik, D., Girelli-Zubani, G., Fiquet, O., Guivel-Benhassine, F., Sanders, R.W., Walker, B.D., Schwartz, O., Scheid, J.F., Dimitrov, J.D., Plevka, P., Braibant, M., Seaman, M.S., Bontems, F., Di Santo, J.P., Rey, F.A., Mouquet, H.(2022) J Exp Medicine 219
- PubMed: 35230385
- DOI: https://doi.org/10.1084/jem.20212045
- Primary Citation of Related Structures:
7PC2 - PubMed Abstract:
Decrypting the B cell ontogeny of HIV-1 broadly neutralizing antibodies (bNAbs) is paramount for vaccine design. Here, we characterized IgA and IgG bNAbs of three distinct B cell lineages in a viremic controller, two of which comprised only IgG+ or IgA+ blood memory B cells; the third combined both IgG and IgA clonal variants. 7-269 bNAb in the IgA-only lineage displayed the highest neutralizing capacity despite limited somatic mutation, and delayed viral rebound in humanized mice. bNAbs in all three lineages targeted the N332 glycan supersite. The 2.8-Å resolution cryo-EM structure of 7-269-BG505 SOSIP.664 complex showed a similar pose as 2G12, on an epitope mainly composed of sugar residues comprising the N332 and N295 glycans. Binding and cryo-EM structural analyses showed that antibodies from the two other lineages interact mostly with glycans N332 and N386. Hence, multiple B cell lineages of IgG and IgA bNAbs focused on a unique HIV-1 site of vulnerability can codevelop in HIV-1 viremic controllers.
Organizational Affiliation:
Laboratory of Humoral Immunology, Department of Immunology, Institut Pasteur, Paris, France.