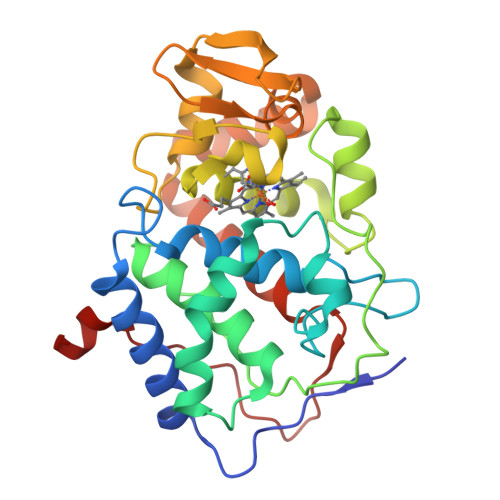
2.2 A structure of oxy-peroxidase as a model for the transient enzyme: peroxide complex.
Miller, M.A., Shaw, A., Kraut, J.(1994) Nat Struct Biol 1: 524-531
- PubMed: 7664080
- DOI: https://doi.org/10.1038/nsb0894-524
- Primary Citation of Related Structures:
1DCC - PubMed Abstract:
The Fe+3-OOH complex of peroxidases has a very short half life, and its structure cannot be determined by conventional methods. The Fe+2-O2 complex provides a useful structural model for this intermediate, as it differs by only one electron and one proton from the transient Fe+3-OOH complex. We therefore determined the crystal structure of the Fe+2-O2 complex formed by a yeast cytochrome c peroxidase mutant with Trp 191 replaced by Phe. The refined structure shows that dioxygen can form a hydrogen bond with the conserved distal His residue, but not with the conserved distal Arg residue. When the transient Fe+3-OOH complex is modelled in a similar orientation, the active site of peroxidase appears to be optimized for catalysing proton transfer between the vicinal oxygen atoms of the peroxy-anion.
Organizational Affiliation:
Department of Chemistry, University of California, San Diego La Jolla 92093-0317.