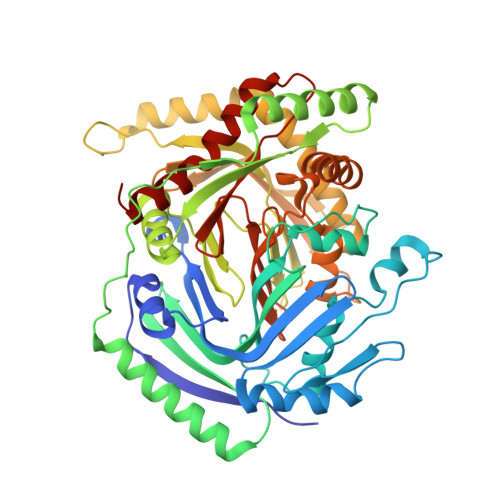
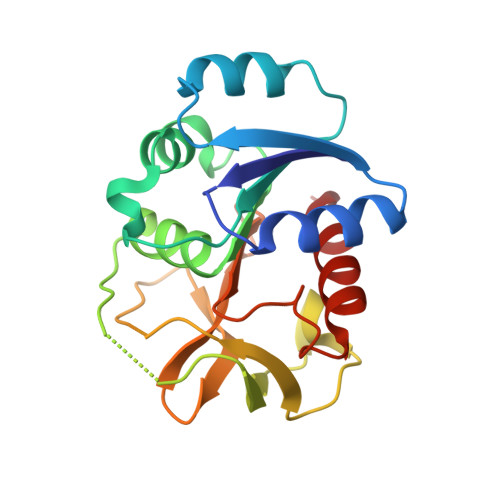
Structure of the cooperative allosteric anthranilate synthase from Salmonella typhimurium.
Morollo, A.A., Eck, M.J.(2001) Nat Struct Biol 8: 243-247
- PubMed: 11224570
- DOI: https://doi.org/10.1038/84988
- Primary Citation of Related Structures:
1I1Q - PubMed Abstract:
We have determined the X-ray crystal structure of the cooperative anthranilate synthase heterotetramer from Salmonella typhimurium at 1.9 A resolution with the allosteric inhibitor l-tryptophan bound to a regulatory site in the TrpE subunit. Tryptophan binding orders a loop that in turn stabilizes the inactive T state of the enzyme by restricting closure of the active site cleft. Comparison with the structure of the unliganded, noncooperative anthranilate synthase heterotetramer from Sulfolobus solfataricus shows that the two homologs have completely different quarternary structures, even though their functional dimer pairs are structurally similar, consistent with differences in the cooperative behavior of the enzymes. The structural model rationalizes mutational and biochemical studies of the enzyme and establishes the structural differences between cooperative and noncooperative anthranilate synthase homologs.
Organizational Affiliation:
Department of Cancer Biology, Dana-Farber Cancer Institute, 44 Binney Street, Boston, Massachusetts 02115, USA.