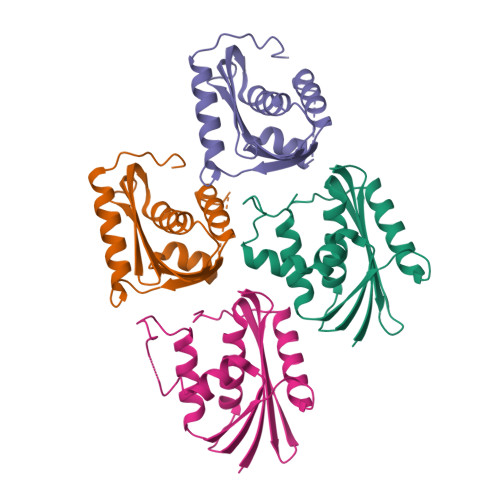
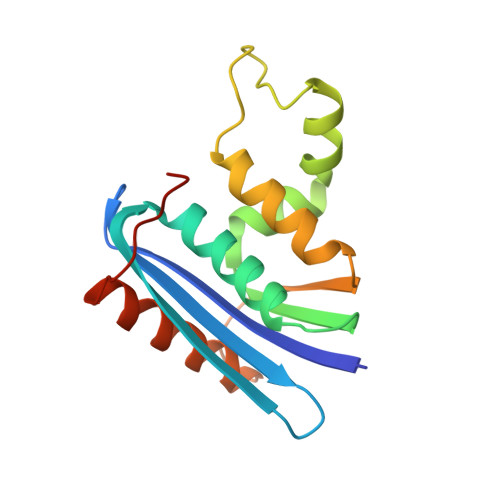
Contributions of folding cores to the thermostabilities of two ribonucleases H.
Robic, S., Berger, J.M., Marqusee, S.(2002) Protein Sci 11: 381-389
- PubMed: 11790848
- DOI: https://doi.org/10.1110/ps.38602
- Primary Citation of Related Structures:
1JL2 - PubMed Abstract:
To investigate the contribution of the folding cores to the thermodynamic stability of RNases H, we used rational design to create two chimeras composed of parts of a thermophilic and a mesophilic RNase H. Each chimera combines the folding core from one parent protein and the remaining parts of the other. Both chimeras form active, well-folded RNases H. Stability curves, based on CD-monitored chemical denaturations, show that the chimera with the thermophilic core is more stable, has a higher midpoint of thermal denaturation, and a lower change in heat capacity (DeltaCp) upon unfolding than the chimera with the mesophilic core. A possible explanation for the low DeltaCp of both the parent thermophilic RNase H and the chimera with the thermophilic core is the residual structure in the denatured state. On the basis of the studied parameters, the chimera with the thermophilic core resembles a true thermophilic protein. Our results suggest that the folding core plays an essential role in conferring thermodynamic parameters to RNases H.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California, Berkeley, California 94720, USA.