Key role of phenylalanine 20 in cytochrome c3: structure, stability, and function studies.
Dolla, A., Arnoux, P., Protasevich, I., Lobachov, V., Brugna, M., Giudici-Orticoni, M.T., Haser, R., Czjzek, M., Makarov, A., Bruschi, M.(1999) Biochemistry 38: 33-41
- PubMed: 9890880
- DOI: https://doi.org/10.1021/bi981593h
- Primary Citation of Related Structures:
1MDV - PubMed Abstract:
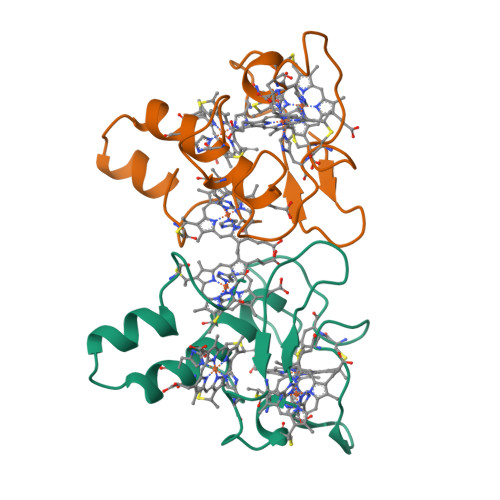
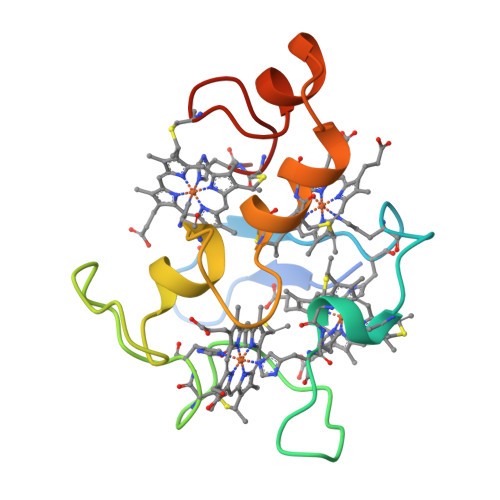
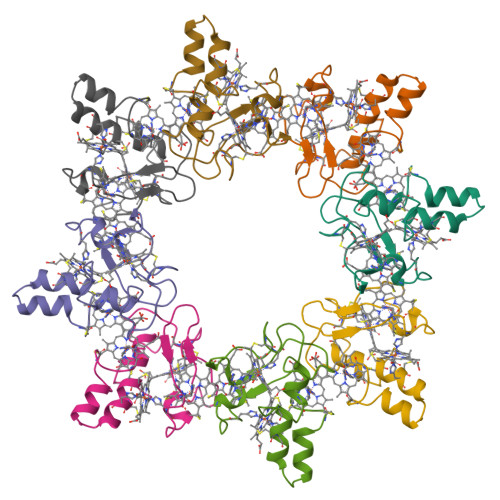
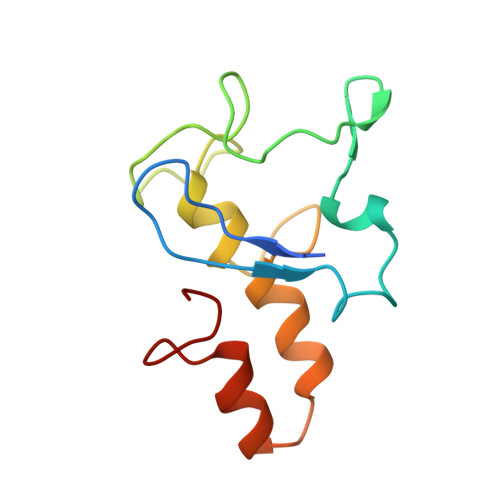
Aromatic residues in c-type cytochromes might have an important function in the folding and/or electron transferring properties of the molecule. In the tetraheme cytochrome c3 (Mr 13 000) from Desulfovibrio vulgaris Hildenborough, Phe20, is located between heme 1 and heme 3 with its aromatic ring close and almost parallel to the ring plane of heme 1. We replaced this residue by a nonaromatic hydrophobe residue, leucine, and analyzed the effects in terms of functional, structural, and physicochemical properties. While the F20L replacement did not have any strong effects on the heme region stability, a decrease of the thermostability of the whole molecule was observed. In the same way, the four macroscopic redox potentials were affected by the mutation as well as the flexibility of the surface loop around heme 4. The F20L replacement itself and/or this structural modification might be responsible for the loss of the intermolecular cooperativity between F20L cytochrome c3 molecules.
Organizational Affiliation:
Laboratoire de Bioénergétique et Ingénierie des protéines, UPR 9036 C.N.R.S., Marseille, France.