Structure of the topoisomerase VI-B subunit: implications for type II topoisomerase mechanism and evolution
Corbett, K.D., Berger, J.M.(2003) EMBO J 22: 151-163
- PubMed: 12505993
- DOI: https://doi.org/10.1093/emboj/cdg008
- Primary Citation of Related Structures:
1MU5, 1MX0 - PubMed Abstract:
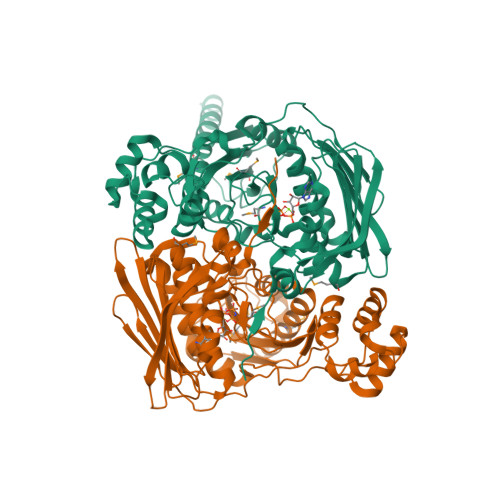
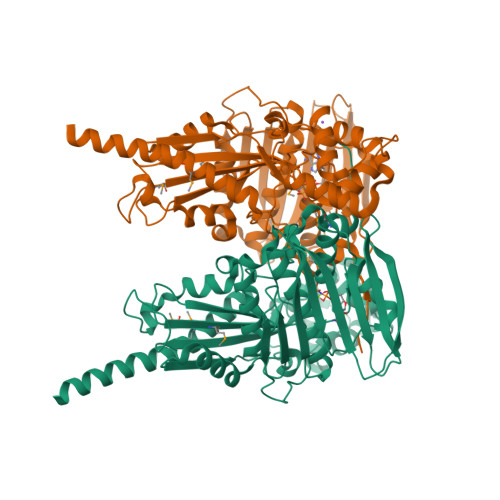
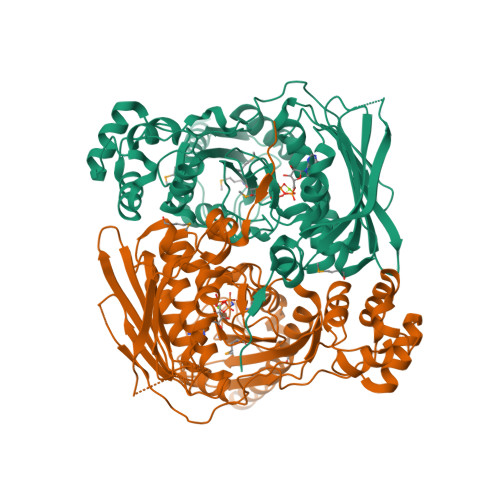
Type IIA and type IIB topoisomerases each possess the ability to pass one DNA duplex through another in an ATP-dependent manner. The role of ATP in the strand passage reaction is poorly understood, particularly for the type IIB (topoisomerase VI) family. We have solved the structure of the ATP-binding subunit of topoisomerase VI (topoVI-B) in two states: an unliganded monomer and a nucleotide-bound dimer. We find that topoVI-B is highly structurally homologous to the entire 40-43 kDa ATPase region of type IIA topoisomerases and MutL proteins. Nucleotide binding to topoVI-B leads to dimerization of the protein and causes dramatic conformational changes within each protomer. Our data demonstrate that type IIA and type IIB topoisomerases have descended from a common ancestor and reveal how ATP turnover generates structural signals in the reactions of both type II topoisomerase families. When combined with the structure of the A subunit to create a picture of the intact topoisomerase VI holoenzyme, the ATP-driven motions of topoVI-B reveal a simple mechanism for strand passage by the type IIB topoisomerases.
Organizational Affiliation:
Department of Molecular and Cellular Biology, University of California, Berkeley, 327 Hildebrand Hall 3206, Berkeley, CA 94720, USA.