Crystal Structure of the A Domain from Complement Factor B Reveals an Integrin-like Open Conformation.
Bhattacharya, A.A., Lupher Jr., M.L., Staunton, D.E., Liddington, R.C.(2004) Structure 12: 371-378
- PubMed: 15016353
- DOI: https://doi.org/10.1016/j.str.2004.02.012
- Primary Citation of Related Structures:
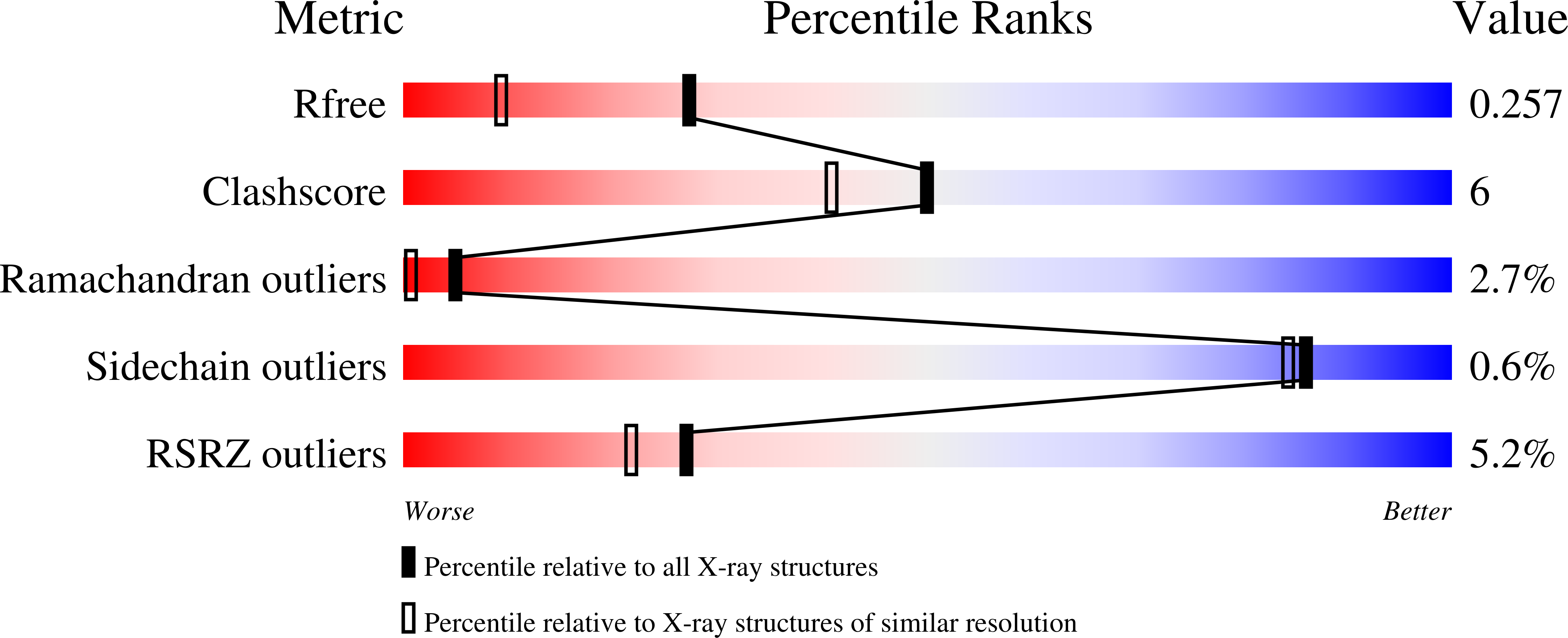
1Q0P - PubMed Abstract:
Complement factor B is a 90 kDa protein consisting of three domains: a three-module complement control protein, a von Willebrand factor A domain, and a C-terminal serine protease (SP) domain that adopts a default inactive (zymogen) conformation. The interaction between factor B and pathogen-bound C3b is mediated by its A domain, triggering a conformational change in factor B that ultimately creates the "C3 convertase" of the alternative complement pathway. We report the crystal structure of the A domain from factor B and show that it contains an integrin-like MIDAS motif that adopts the "open" conformation typical of integrin-ligand complexes, with an acidic residue (provided by a fortuitous crystal contact) completing the coordination of the metal ion. Modeling studies indicate that the factor B A domain can also adopt the closed conformation, supporting the hypothesis that an "integrin-like switch" is conserved in complement proteins and perhaps in 60 other A domains found within the human proteome.
Organizational Affiliation:
The Burnham Institute, 10901 North Torrey Pines Road, La Jolla, CA 92037 USA.