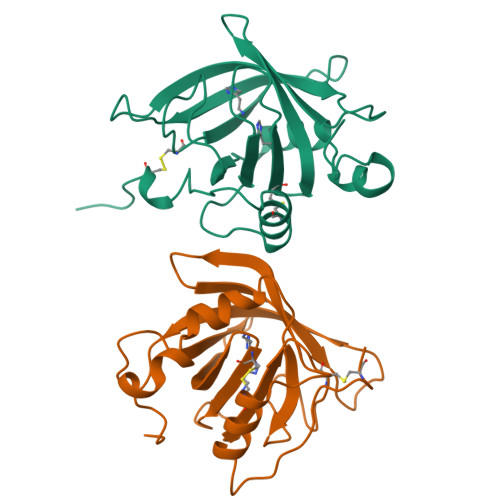
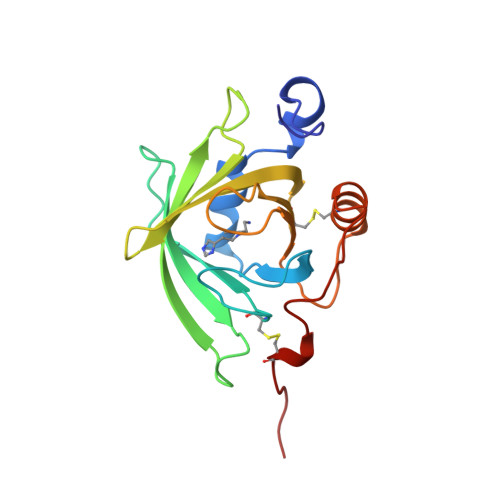
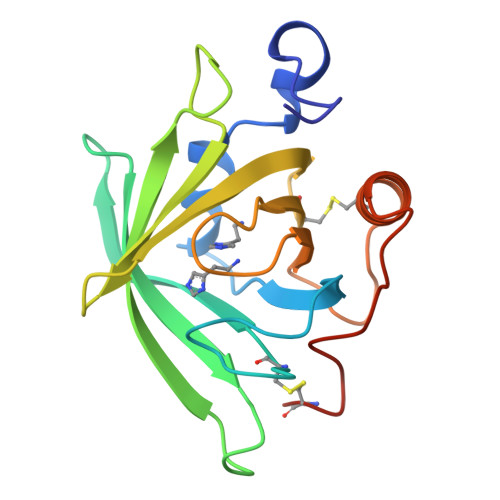
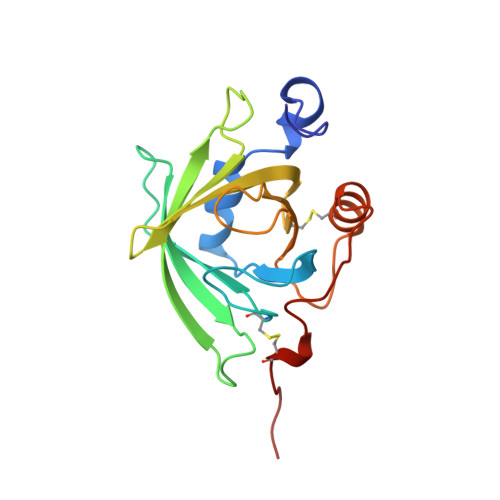
Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure.
Paesen, G.C., Adams, P.L., Harlos, K., Nuttall, P.A., Stuart, D.I.(1999) Mol Cell 3: 661-671
- PubMed: 10360182
- DOI: https://doi.org/10.1016/s1097-2765(00)80359-7
- Primary Citation of Related Structures:
1QFT, 1QFV - PubMed Abstract:
High-affinity histamine-binding proteins (HBPs) were discovered in the saliva of Rhipicephalus appendiculatus ticks. Their ability to outcompete histamine receptors indicates that they suppress inflammation during blood feeding. The crystal structure of a histamine-bound HBP, determined at 1.25 A resolution, reveals a lipocalin fold novel in containing two binding sites for the same ligand. The sites are orthogonally arranged and highly rigid and form an internal surface of unusual polar character that complements the physicochemical properties of histamine. As soluble receptors of histamine, HBPs offer a new strategy for controlling histamine-based diseases.
Organizational Affiliation:
Natural Environment Research Council, Institute of Virology and Environmental Microbiology, Oxford, United Kingdom. gcp@mail.nerc-oxford.ac.uk