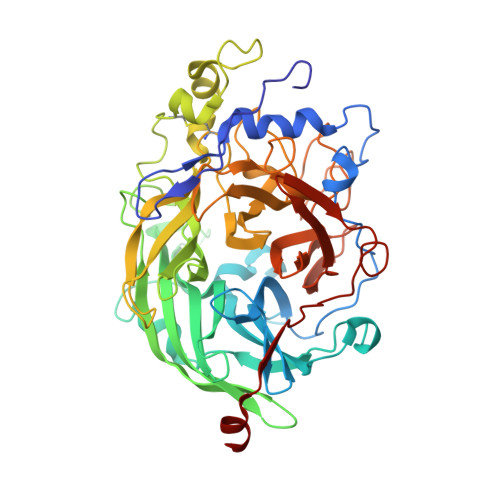
Crystal Structure of Levansucrase from the Gram- Negative Bacterium Gluconacetobacter Diazotrophicus.
Martinez-Fleites, C., Ortiz-Lombardia, M., Pons, T., Tarbouriech, N., Taylor, E.J., Arrieta, J.G., Hernandez, L., Davies, G.J.(2005) Biochem J 390: 19
- PubMed: 15869470
- DOI: https://doi.org/10.1042/BJ20050324
- Primary Citation of Related Structures:
1W18 - PubMed Abstract:
The endophytic Gram-negative bacterium Gluconacetobacter diazotrophicus SRT4 secretes a constitutively expressed levansucrase (LsdA, EC 2.4.1.10), which converts sucrose into fructooligosaccharides and levan. The enzyme is included in GH (glycoside hydrolase) family 68 of the sequence-based classification of glycosidases. The three-dimensional structure of LsdA has been determined by X-ray crystallography at a resolution of 2.5 A (1 A=0.1 nm). The structure was solved by molecular replacement using the homologous Bacillus subtilis (Bs) levansucrase (Protein Data Bank accession code 1OYG) as a search model. LsdA displays a five-bladed beta-propeller architecture, where the catalytic residues that are responsible for sucrose hydrolysis are perfectly superimposable with the equivalent residues of the Bs homologue. The comparison of both structures, the mutagenesis data and the analysis of GH68 family multiple sequences alignment show a strong conservation of the sucrose hydrolytic machinery among levansucrases and also a structural equivalence of the Bs levansucrase Ca2+-binding site to the LsdA Cys339-Cys395 disulphide bridge, suggesting similar fold-stabilizing roles. Despite the strong conservation of the sucrose-recognition site observed in LsdA, Bs levansucrase and GH32 family Thermotoga maritima invertase, structural differences appear around residues involved in the transfructosylation reaction.
Organizational Affiliation:
Structural Biology Laboratory, Chemistry Department, University of York, York YO10 5YW, UK. martinez@ysbl.york.ac.uk